Abstract
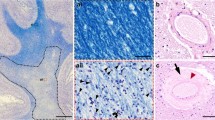
Amyotrophic lateral sclerosis (ALS) is associated with frontotemporal lobar degeneration (FTLD) in 15% of the cases. A neuropathological continuity between ALS and FTLD-TDP is suspected. The present post-mortem 7.0-tesla magnetic resonance imaging (MRI) study compares the topographic distribution of iron (Fe) deposition and the incidence of small cerebrovascular lesions in ALS and in FTLD brains. Seventy-eight post-mortem brains underwent 7.0-tesla MRI. The patients consisted of 12 with ALS, 38 with FTLD, and 28 controls. Three ALS brains had minor FTLD features. Three coronal sections of a cerebral hemisphere were submitted to T2 and T2* MRI sequences. The amount of Fe deposition in the deep brain structures and the number of small cerebrovascular lesions was determined in ALS and the subtypes of FTLD compared to control brains, with neuropathological correlates. A significant increase of Fe deposition was observed in the claustrum, caudate nucleus, globus pallidus, thalamus, and subthalamic nucleus of the FTLD-FUS and FTLD-TDP groups, while in the ALS one, the Fe increase was only observed in the caudate and the subthalamic nuclei. White matter changes were only significantly more severe in the FTLD compared to those in ALS and in controls brains. Cortical micro-bleeds were increased in the frontal and temporal lobes of FTLD as well as of ALS brains compared to controls. Cortical micro-infarcts were, on the other hand, more frequent in the control compared to the ALS and FTLD groups. The present study supports the assumption of a neuropathological continuity between ALS and FTLD and illustrates the favourable vascular risk profile in these diseases.


Similar content being viewed by others
References
Liscic RM, Grinberg LT, Zidar J, Gitcho MA, Cairns NJ (2008) ALS and FTLD: two faces of TDP-43 proteinopathy. Eur J Neurol 15:772–780
Sudedja NA, van der Schouw YT, Fischer K, Sizoo EM, Huisman MH, Veldink JH et al (2011) Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 8:638–642
Kioumourtzoglou MA, Rotem RS, Seals RM, Gredal O, Hansen J, Weisskopf MG (2015) Diabetes, obesity, and diagnosis of amyotrophic lateral sclerosis: a population based study. JAMA Neurol 72:905–911
Sieben A, Van Langenhove T, Engelborghs S, Martin JJ, Boon P, Cras P et al (2012) The genetic and neuropathology of frontotemporal lobar degeneration. Acta Neuropathol 124:353–372
De Reuck J, Deramecourt V, Cordonnier C, Auger F, Durieux N, Bordet R et al (2012) Detection of microbleeds in post-mortem brains of patients with frontotemporal lobar degeneration: a 7.0-tesla magnetic resonance imaging study with neuropathological correlates. Eur J Neurol 19:1355–1360
De Reuck J, Auger F, Durieux N, Deramecourt V, Maurage CA, Lebert F et al (2016) The topography of cortical microbleeds in frontotemporal lobar degeneration: a post-mortem 7.0-tesla magnetic resonance study. Folia Neuropathol 54:149–155
Ding B, Chen KM, Ling HW, Sun F, Li X, Wan T et al (2009) Correlation of iron in the hippocampus with MMSE in patients with Alzheimer’s disease. J Magn Reson Imaging 29:793–798
Del Valdes Hernandez M, Ritchie S, Glatz A, Allerhand M, Munos Maniega S, Gow AJ et al (2015) Brain iron deposits and lifespan cognitive ability. Age 37(5):100
De Reuck J, Deramecourt V, Auger F, Durieux N, Cordonnier C, Devos D et al (2014) Iron deposits in post-mortem brains of patients with neurodegenerative and cerebrovascular diseases: a semi-quantitative 7.0 T magnetic resonance imaging study. Eur J Neurol 21:1026–1031
Veyrat-Durebex C, Corcia P, Mucha A, Benzimra S, Mallet C, Gendrot C et al (2014) Iron metabolism disturbance in a French cohort of ALS patients. Biomed Res Int. doi:10.1155/2014/485723
Kasarskis EJ, Tandon L, Lovell MA, Ehmann WD (1995) Aluminium, calcium, and iron in the spinal cord of patients with sporadic amyotrophic lateral sclerosis using laser microprobe mass spectroscopy: a preliminary study. J Neurol Sci 130:203–208
Igjatovic A, Stevic Z, Lavrnic S, Dakovic M, Bacic G (2013) Brain iron MRI: a biomarker for amyotrophic lateral sclerosis. J Magn Reson Imaging 38:1472–1479
Costagli M, Donatelli G, Biagi L, Caldarazzo Lenco E, Siciliano Tosetti M et al (2016) Magnetic susceptibility in the deep layers of the primary motor cortex in amyotrophic lateral sclerosis. Neuroimage Clin 12:965–969
Hecht MJ, Fellner C, Schmid A, Neundorfer B, Fellner FA (2005) Cortical T2 signal shortening in amyotrophic lateral sclerosis is not due to iron deposits. Neuroradiology 47:805–808
Cruz-Sanchez FF, Moral A, de Belleroche J, Rossi ML (1993) Amyotrophic lateral sclerosis brain banking: a proposal to standardize protocols and neuropathological diagnostic criteria. J Neural Transm 39(Suppl 2):215–222
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ et al (2007) Consortium for Frontotemporal Lobar Degeneration. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22
De Reuck J, Deramecourt V, Cordonnier C, Leys D, Pasquier F, Maurage C-A (2011) Prevalence of small cerebral bleeds in patients with a neurodegenerative dementia: a neuropathological study. J Neurol Sci 300:63–66
De Reuck J, Auger F, Cordonnier C, Deramecourt V, Durieux N, Pasquier F et al (2011) Comparison of 7.0-Tesla T2*-magnetic resonance imaging of cerebral bleeds in post-mortem brain sections of Alzheimer patients with their neuropathological correlates. Cerebrovasc Dis 31:511–517
Meadowcroft MD, Connor JR, Smith MB, Yang QX (2009) MRI and histological analysis of bête-amyloid plaques in both human Alzheimer’s disease and APP/PS1 transgenetic mice. J Magn Reson Imaging 29:997–1007
Gupta D, Saini J, Kesavadas C, Sarma S, Kishore A (2010) Utility of susceptibility-weighted MRI in differentiating Parkinson’s disease and atypical parkinsonism. Neuroradiology 52:1087–1094
Hopes L, Grolez G, Moreau C, Lopes R, Ryckewaert G, Carnière N et al (2016) Magnetic resonance imaging features of the nigrostriatal system: biomarkers of Parkinson’s disease stages? PLoS ONE 16:e0147947
Ehmann WD, Alauddin M, Hossain TI, Markesbery WR (1984) Brain trace elements in Pick’s disease. Ann Neurol 15:102–104
Santillo AF, Skoglund L, Lindau M, Eeg-Olofsson KE, Tovi M, Engler H et al (2009) Frontotemporal dementia-amyotrophic lateral sclerosis complex is simulated by neurodegeneration with brain iron accumulation. Alzheimer Dis Assoc Disord 23:298–300
Jeong SY, Rathore KI, Schulz K, Ponka P, Arosio P, Davis S (2009) Dysregulation of iron homeostasis in the CNS contributes to disease progression in a mouse model of amyotrophic lateral sclerosis. J Neurosci 29:610–619
Prpar Mihevc S, Darovic S, Kovanda A, Bajc Cesnik A, Zupunski V, Rogeli B (2017) Nuclear trafficking in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Brain 140:13–26
Gazzina S, Premi E, Zanella I, Biasiotto G, Archetti S, Cosseddu M et al (2016) Iron in frontotemporal lobar degeneration A new subcortical pathological pathway? Neurodegener Dis 16:172–178
Grolez G, Moreau C, Daniel-Brunaud V, Delmaire C, Lopes R, Pradat PF et al (2016) The value of magnetic resonance imaging as a biomarker of amyotrophic lateral sclerosis: a systematic review. BMC Neurol 16:155. doi:10.1186/s12883-016-0672-6
Agosta F, Galantucci S, Magnani G, Marcone A, Martinelli D, Antonietta Volontè M et al (2015) MRI signatures of the frontotemporal lobar degeneration continuum. Hum Brain Mapp 36:2602–2614
Moreau C, Brunaud-Daniel V, Dallongeville J, Duhamel A, Laurier- Grymonprez L, De Reuck J et al (2011) Modifying effect of arterial hypertension on amyotrophic lateral sclerosis. Amyotroph Lateral Scler 13:194–202
Riku Y, Watanabe H, Yoshida M, Tatsumi S, Mimuro M, Iwasaki M et al (2014) Lower motor neuron involvement in TAR DNA-binding protein of 43 kDa-related frontotemporal lobar degeneration and amyotrophic lateral sclerosis. JAMA Neurol 71:172–179
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
The brain tissue samples were acquired from the Lille Neuro-Bank of the Lille University, federated to the “Centre des Resources Biologiques” that acted as an institutional review board.
Informed consent
Previously obtained informed consent of the patients or from the nearest family allowed an autopsy for diagnostic and scientific purposes.
Rights and permissions
About this article
Cite this article
De Reuck, J., Devos, D., Moreau, C. et al. Topographic distribution of brain iron deposition and small cerebrovascular lesions in amyotrophic lateral sclerosis and in frontotemporal lobar degeneration: a post-mortem 7.0-tesla magnetic resonance imaging study with neuropathological correlates. Acta Neurol Belg 117, 873–878 (2017). https://doi.org/10.1007/s13760-017-0832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-017-0832-5