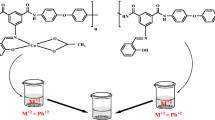
Abstract
In this study, first a diacid Schiff base ligand was synthesized via the reaction of 2-hydroxybenzaldehyde with 5-aminoisophthalic acid. Then, Schiff base nanostructure aromatic polyamide was prepared by the reaction of 4,4′-diaminodiphenyl ether and the synthesized diacid in the presence of molten tetrabutylammonium bromide (TBAB)/triphenyl phosphite (TPP) as the green reaction medium. Then, the metal-polymer complex was synthesized from polyamide ligand and Manganese(III) acetate dihydrate salt. The structure, optical, the thermal and morphological properties of these compounds were confirmed by FT-IR, 1H NMR, elemental analysis, UV-Vis, powder X-ray diffraction technique (XRD), thermogravimetric analysis (TGA), and field emission scanning electron microscopy (FE-SEM) techniques. The nanostructure polyamide complex showed a good adsorption behavior for the elimination of Cd(II) and Hg(II) ions from aquatic solutions; it was also easy to recover and reuse. The sample achieved equilibrium in 30 min at the pH of 7. The adsorption data of Hg(II) and Cd(II) ions were fitted to the Langmuir and Freundlich models, respectively. Furthermore, a pseudo-second-order rate pattern was adapted to the kinetic data of Hg(II) and Cd(II) ions.
Graphic abstract











Similar content being viewed by others
References
H.H. Savenije, Phys. Chem. Earth 27, 741 (2002)
A. Bhatnagar, M. Sillanpää, Chem. Eng. J. 157, 277 (2010)
H.P. Srivastava, G. Arthanareeswaran, N. Anantharaman, V.M. Starov, Desalination 282, 87 (2011)
G. Arthanareeswaran, V.M. Starov, Desalination 267, 57 (2011)
M.A. Hashim, S. Mukhopadhyay, J.N. Sahu, B. Sengupta, J. Environ. Manage. 92, 2355 (2011)
F. Wang, X. Lu, X.Y. Li, J. Hazard. Mater. 308, 75 (2016)
S.K. Bozbas, U. Ay, A. Kayan, Desal. Wat. Treat. 51, 7208 (2013)
J. Shao, J.D. Gu, L. Peng, S. Luo, H. Luo, Z. Yan, G. Wu, J. Hazard. Mater. 272, 83 (2014)
N. Kallithrakas-Kontos, S. Foteinis, Curr. Anal. Chem. 12, 22 (2016)
F. Fu, Q. Wang, J. Environ. Manage. 92, 407 (2011)
M. Feizi, M. Jalali, J. Taiwan Inst. Chem. Eng. 54, 125 (2015)
M. Dinari, G. Mohammadnezhad, R. Soltani, RSC Adv. 6, 11419 (2016)
A. Li, R. Lin, C. Lin, B. He, T. Zheng, L. Lu, Y. Cao, Carbohydr. Polym. 148, 272 (2016)
G. Mohammadnezhad, M. Dinari, R. Soltani, New J. Chem. 40, 3612 (2016)
B. Samiey, C.H. Cheng, J. Wu, Materials 7, 673 (2014)
S.S. Gupta, K.G. Bhattacharyya, J. Hazard. Mater. 128, 247 (2006)
Y.S. Ok, J.E. Yang, Y.S. Zhang, S.J. Kim, D.Y. Chung, J. Hazard. Mater. 147, 91 (2007)
M.R. Awual, Chem. Eng. J. 307, 85 (2017)
H. Li, Y. Ge, X. Zhang, A. Colloids, Surf. Physicochem. Eng. Aspects 513, 306 (2017)
I. Craciunescu, A. Petran, J. Liebscher, L. Vekas, R. Turcu, Mater. Chem. Phys. 185, 91 (2017)
M. Aiba, T. Higashihara, M. Ashizawa, H. Otsuka, H. Matsumoto, Macromolecules. 49, 2153 (2016)
S. Banerjee, S. Maji, High-performance processable aromatic polyamides, in High performance polymers and engineering plastics, ed. by V. Mittal (Wiley, Hoboken, 2011), p. 111
J.M. García, F.C. García, F. Serna, L. José, Prog. Polym. Sci. 35, 623 (2010)
A. Iwan, D. Sek, Prog. Polym. Sci. 33, 289 (2008)
A. Schmidt, A. Beutler, M. Albrecht, B. Snovydovych, F.J. Ramírez, Org. Biomol. Chem. 6, 287 (2008)
S. Mallakpour, M. Dinari, Macromol. Res. 18, 129 (2010)
S. Mallakpour, M. Dinari, J. Polym. Environ. 18, 705 (2010)
S. Mallakpour, M. Dinari, Iran. Polym. J. 19, 983 (2010)
M.A. Fardjahromi, M. Moghadam, S. Tangestaninejad, V. Mirkhani, I. Mohammadpoor-Baltork, RSC Adv. 6, 20128 (2016)
V. Mirkhani, M. Moghadam, S. Tangestaninejad, B. Bahramian, Appl. Catal. A Gen. 311, 43 (2006)
L.J. Zhang, L. Qi, X.Y. Chen, F. Liu, L.J. Liu, W.L. Ding, D.L. Li, G.C. Yuan, J.Z. Tong, F.Y. Chen, H.J. Huang, Y.H. Wang, J. Chem. Crystallogr. (2018). https://doi.org/10.1007/s10870-018-0761-z
M. Dinari, A. Haghighi, Prog. Org. Coat. 110, 24 (2017)
R. Rasool, S. Hasnaina, N. Nishata, Des. Monomers Polym. 17, 217 (2014)
D.J. Darensbourg, E.B. Frantz, Inorg. Chem. 46, 5967 (2007)
F. Chioma, A.C. Ekennia, A.A. Osowole, S.N. Okafor, C.U. Ibeji, D.C. Onwudiwe, O.T. Ujam, Open Chem. 16, 184 (2018)
S. Kumar, R.R. Jha, S. Yadav, R. Gupta, New J. Chem. 39, 2042 (2015)
M. Tyagi, S. Chandra, J. Saudi. Chem. Soc. 18, 53 (2014)
S.Y. Ebrahimipour, J.T. Mague, A. Akbari, R. Takjoo, J. Mol. Struct. 1028, 148 (2012)
Acknowledgements
The authors are thankful to the credits of Isfahan University of Technology (IUT) for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declared that they have no conflicts of interest in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aghaei, M., Kianfar, A.H. & Dinari, M. Green synthesis of nanostructure Schiff base complex based on aromatic polyamide and manganese(III) for elimination of Hg(II) and Cd(II) from solutions. J IRAN CHEM SOC 16, 2489–2500 (2019). https://doi.org/10.1007/s13738-019-01719-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01719-x