Abstract
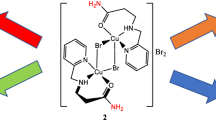
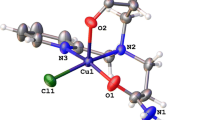
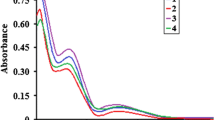
The synthesis of two mononuclear copper(II) complexes of formula [L2CuX]X where L is a bidentate ligand of N-(pyridin-2-yl-methyl)propane-2-amine and X = Cl, 1 and Br, 2, is reported. Both complexes are fully characterized by elemental analysis, spectroscopic techniques (IR, UV–Vis, and EPR), thermal analysis, conductance measurements, and single-crystal X-ray structure determination. The structures of both complexes are similar and display a distorted square–pyramidal arrangement around copper(II) ion. The chromotropism behavior of the complexes, including thermo- and halochromism, was investigated. The complexes demonstrated strongly pronounced reversible thermochromism in solution due to dissociation and re-coordination of halide and bidentate ligands. Their halochromism was investigated in pH range of 3.0–11.0 by visible absorption spectroscopy. Their reversible color variations from blue to colorless attributed to the deprotonation and protonation of the bidentate ligands.











Similar content being viewed by others
References
W. Linert. Y. Fukuda, A. Camard, Coord. Chem. Rev. 218, 113–152 (2001)
Y. Fukuda, Inorganic chromotropism Springer, Berlin, 2007
C.F. Zhu, A.B. Wu, Thermochim. Acta 425, 7–12 (2005)
A. Hamberger, A.M. Popa, R.M. Rossi, D.R. Kattnig, D. Hinderberger, K. Landfester, D. Crespy, J. Mater. Chem. 22, 9909–9920 (2012)
V.N. Khrustalev, S.O. Kostenko, M.I. Buzin, A.A. Korlyukov, Y.V. Zubavichus, M.A. Kurykin, M.Y. Antipin, Inorg. Chem. 51, 10590–10602 (2012)
H. Golchoubian, L. Rostami, J. Coord. Chem. 70, 3660–3676 (2017)
L. Stryer, Biochemistry, 3rd Ed. New York: Freeman; 1988
F. Cheng, N. Tang, J. Chen, F. Wang, L. Chen, Inorg. Chem. Commun. 14, 852–855 (2011)
P. Bamfield, Chromic phenomena: technological applications of color chemistry Springer, Berlin, 2002, p. 8
H. Golchoubian, E. Rezaee, G. Bruno, H.A. Rudbari, Inorg. Chim. Acta 366, 290–297 (2011)
H. Golchoubian, R. Samimi, Polyhedron 128, 68–75 (2017)
H. Golchoubian, M. Tarahomi, E. Rezaee, G. Bruno, Polyhedron 85, 635–642 (2015)
SAINT, Software for the CCD Detector System, (version 7.06A), Bruker AXS (Inc, Madison, Wisconsin, 2005)
M.C. Burla, R. Caliandro, M. Camalli, B. Carrozzini, G.L. Cascarano, L. De Caro, C. Giacovazzo, G. Polidori, R. Spagna, J. Appl. Cryst. 38, 381–388 (2005)
G.M. Sheldrick, SHELXL-2014/7: program for the solution of crystal structures (University of Göttingen, Göttingen, 2014)
G.M. Sheldrick, Acta Cryst. C71, 3–8 (2015)
A.W. Addison, T.N. Rao, J. Reedijk, J. van Rijn, G.C. Verschoor, J. Chem. Soc. Dalton Trans. 1349–1356 (1984)
S.Q. Bai, E.Q. Gao, Z. He, C.J. Fang, C.H. Yan, New J. Chem. 29, 935–941 (2005)
T. Pandiyan, H.J. Guadalupe, J. Cruz, S. Bernès, V.M. Ugalde-Salvdivar, Eur. J. Inorg. Chem. 21, 3274–3285 (2008)
Cambridge Structural Database System, Cambridge Crystallographic Data Centre, University Chemical Laboratory, Cambridge, UK
C. Tsiamis, M. Themeli, Inorg. Chim. Acta 206, 105–115 (1993)
W.J. Geary, Coord. Chem. Rev. 7, 81–122 (1971)
C.J. Williams, H. Morris, J. Svorec, M. Valková, M. Valko, J. Moncol, M. Mazur, F. Valach, M. Melnik, J. Mol. Struct. 659, 53–60 (2003)
V. Sakaguchi, A.W. Addison, J. Chem. Soc. Dalton Trans. 600–608 (1979)
J. Losada, I. Del Peso, L. Beyer, Inorg. Chim. Acta 321, 107–115 (2001)
D. Kivelson, R. Neiman, J. Chem. Phys. 35, 149–155 (1961)
Acknowledgements
We are grateful for the financial support of the University of Mazandaran of the Islamic Republic of Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nazari, R., Golchoubian, H. & Bruno, G. Mononuclear copper(II) complexes containing chelating ligand of 2-methyl-N-(pyridine-2-yl-methyl)propane-2-amine as chromotropic probes. J IRAN CHEM SOC 16, 1041–1052 (2019). https://doi.org/10.1007/s13738-018-01577-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-01577-z