Abstract
In this study, some novel 4-thiazolylpyrazoles were synthesized by modified Hantzsch method, under solvent-free conditions and in the presence of MgO nanoparticles as catalyst. Excellent yields in shorter reaction times were achieved under the new general protocol. Also, the in vitro antibacterial effects of synthesized compounds were evaluated against 21 gram-positive and gram-negative pathogenic bacterial strains and compared to antibiotics such as ceftriaxone and penicillin. The results were reported as inhibition zone diameter, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) values. The compounds showed moderate to good antibacterial activities. Among the newly synthesized thiazoles, compounds 7b, f had inhibitory effects against eight pathogenic bacteria; besides, thioamide 6 with MIC and MBC values of 32 and 64 μg/mL against Streptococcus agalactiae was identified as the most effective antibacterial agent.
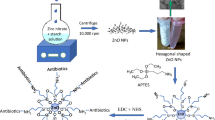
Graphical Abstract
MgO nanoparticle was prepared and successfully used as an efficient recyclable catalyst for the Hantzsch thiazole synthesis; reaction times and product yields were significantly improved in the presence of nanocatalyst.






Similar content being viewed by others
References
A. Ayati, S. Emami, A. Asadipour, A. Shafiee, A. Foroumadi, Eur. J. Med. Chem. 97, 699 (2015)
Z. Xu, T. Ye, in Heterocycles, in Natural Product Synthesis, ed. by K.C. Majumdar, S.K. Chattopadhyay (Wiley-VCH, Weinheim, 2011), pp. 459–505
V. Gupta, V. Kant, Sci. Int. 1, 253 (2013)
F. Sasse, H. Steinmetz, G. Höfle, H. Reichenbach, J. Antibiot. 56, 520 (2003)
M.Y. Kim, H. Vankayalapati, K. Shin-Ya, K. Wierzba, L.H. Hurley, J. Am. Chem. Soc. 124, 2098 (2002)
C.B. Mishra, S. Kumari, M. Tiwari, Eur. J. Med. Chem. 92, 1 (2015)
P.W. Sheldrake, M. Matteucci, E. McDonald, Synlett 2006, 460 (2006)
G.S. Lingaraju, T.R. Swaroop, A.C. Vinayaka, K.S.S. Kumar, M.P. Sadashiva, K.S. Rangappa, Synthesis 44, 1373 (2012)
D. Castagnolo, M. Pagano, M. Bernardini, M. Botta, Synlett 2009, 2093 (2009)
J.F. Sanz-Cervera, R. Blasco, J. Piera, M. Cynamon, I. Ibáñez, M. Murguía, S. Fustero, J. Org. Chem. 74, 8988 (2009)
M. Narender, M.S. Reddy, V.P. Kumar, B. Srinivas, R. Sridhar, Y.V.D. Nageswar, K.R. Rao, Synthesis 2007, 3469 (2007)
Y. Ishiwata, H. Togo, Synlett 2008, 2637 (2008)
U. Kazmaier, S. Ackermann, Org. Biomol. Chem. 3, 3184 (2005)
P.B. Gorepatil, Y.D. Mane, V.S. Ingle, Synlett 24, 2241 (2013)
Y. Sun, H. Jiang, W. Wu, W. Zeng, X. Wu, Org. Lett. 15, 1598 (2013)
T.B. Nguyen, L. Ermolenko, W.A. Dean, A. Al-Mourabit, Org. Lett. 14, 5948 (2012)
T. Guntreddi, R. Vanjari, K.N. Singh, Org. Lett. 17, 976 (2015)
X. Zhang, W. Zeng, Y. Yang, H. Huang, Y. Liang, Org. Lett. 16, 876 (2014)
H. Zheng, Y.J. Mei, K. Du, X.T. Cao, P.F. Zhang, Molecules 18, 13425 (2013)
M. Kodomari, T. Aoyama, Y. Suzuki, Tetrahedron Lett. 43, 1717 (2002)
S.L. You, J.W. Kelly, Tetrahedron 61, 241 (2005)
J. Hämmerle, M. Spina, M. Schnürch, M.D. Mihovilovic, P. Stanetty, Synthesis 2008, 3099 (2008)
H. Beyzaei, R. Aryan, H. Moghadas, J. Serbian Chem. Soc. 80, 453 (2015)
F.L. Chubb, J.T. Edward, Can. J. Chem. 59, 2724 (1981)
S.M. Al-Mousawi, M.S. Moustafa, M.H. Elnagdi, Arkivoc 2010, 224 (2010)
M. Suresh, P. Lavanya, C.V. Rao, Arab. J. Chem. 9, 136 (2016)
J. Banothu, K. Vaarla, R. Bavantula, P.A. Crooks, Chin. Chem. Lett. 25, 172 (2014)
A. Safari, Z. Abedi-Jazini, Z. Zarnegar, M. Sadeghi, J. Nanoparticle Res. 17, 495 (2015)
M.W. Bredenkamp, C.W. Holzapfel, W.J. van Zyl, Syn. Commun. 20, 2235 (1990)
V.E. Bhingolikar, S.R. Mahalle, S.P. Bondge, R.A. Mane, Indian J. Chem. 44B, 2589 (2005)
A.S. Shahvelayati, I. Yavari, A.S. Delbari, Chin. Chem. Lett. 25, 119 (2014)
I. Yavari, A. Malekafzali, S. Seyfi, J. Iran. Chem. Soc. 11, 285 (2014)
H. Zali-Boeini, S.G. Mansouri, J. Iran. Chem. Soc. 13, 1571 (2016)
T.M. Potewar, S.A. Ingale, K.V. Srinivasan, Tetrahedron 63, 11066 (2007)
B.S. Dawane, S.G. Konda, V.T. Kamble, S.A. Chavan, R.B. Bhosale, B.M. Shaikh, Eur. J. Chem. 6, 358 (2009)
S.M. Gomha, K.D. Khalil, Molecules 17, 9335 (2012)
P.S. Shisode, P.P. Mahulikar, J. Chem. Pharm. Res. 2, 576 (2010)
L. Zeng, K. Li, F. Huang, X. Zhu, H. Li, Chin. J. Catal. 37, 908 (2016)
M. Sundrarajan, J. Suresh, R. Gandhi, Dig. J. Nanomater. Biostruct. 7, 983 (2012)
F. Allouche, F. Chabchoub, F. Carta, C.T. Supuran, J. Enzyme Inhib. Med. Chem. 28, 343 (2013)
B. Kaboudin, D. Elhamifar, Synthesis 2006, 224 (2006)
M. Balouiri, M. Sadiki, S.K. Ibnsouda, J. Pharm. Anal. 6, 71 (2016)
P. Giori, A.C. Veronese, C.B. Vicentini, M. Guarneri, J. Heterocycl. Chem. 22, 1093 (1985)
Acknowledgement
The authors would like to thank members of the University of Zabol especially Dr. Mansour Ghaffari-Moghaddam, dean of the faculty of science, for their support and assistance at the various stages of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beyzaei, H., Aryan, R., Molashahi, H. et al. MgO nanoparticle-catalyzed, solvent-free Hantzsch synthesis and antibacterial evaluation of new substituted thiazoles. J IRAN CHEM SOC 14, 1023–1031 (2017). https://doi.org/10.1007/s13738-017-1052-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1052-x