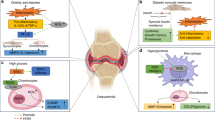
Abstract
Purpose of Review
Metabolic syndrome (MetS), also called the ‘deadly quartet’ comprising obesity, diabetes, dyslipidemia, and hypertension, has been ascertained to have a causal role in the pathogenesis of osteoarthritis (OA). This review is aimed at discussing the current knowledge on the contribution of metabolic syndrome and its various components to OA pathogenesis and progression.
Recent Findings
Lately, an increased association identified between the various components of metabolic syndrome (obesity, diabetes, dyslipidemia, and hypertension) with OA has led to the identification of the ‘metabolic phenotype’ of OA. These metabolic perturbations alongside low-grade systemic inflammation have been identified to inflict detrimental effects upon multiple tissues of the joint including cartilage, bone, and synovium leading to complete joint failure in OA. Recent epidemiological and clinical findings affirm that adipokines significantly contribute to inflammation, tissue degradation, and OA pathogenesis mediated through multiple signaling pathways. OA is no longer perceived as just a ‘wear and tear’ disease and the involvement of the metabolic components in OA pathogenesis adds up to the complexity of the disease.
Summary
Given the global surge in obesity and its allied metabolic perturbations, this review aims to throw light on the current knowledge on the pathophysiology of MetS-associated OA and the need to address MetS in the context of metabolic OA management. Better regulation of the constituent factors of MetS could be profitable in preventing MetS-associated OA. The identification of key roles for several metabolic regulators in OA pathogenesis has also opened up newer avenues in the recognition and development of novel therapeutic agents.

Similar content being viewed by others
References
Jaggard M, et al. Can metabolic profiling provide a new description of osteoarthritis and enable a personalised medicine approach? 2020;39:3875–3882.
Wang H, et al. Metabolic syndrome increases the risk for knee osteoarthritis: a meta-analysis. Evidence-Based Complementary and Alternative Medicine. 2016;2016:7242478.
Liu S-Y, et al. Bidirectional association between metabolic syndrome and osteoarthritis: a meta-analysis of observational studies. Diabetol Metab Syndr. 2020;12(1):38.
Pan F, et al. Association between metabolic syndrome and knee structural change on MRI. Rheumatology. 2019;59(1):185–93.
Jha BK, et al. Progress in understanding metabolic syndrome and knowledge of its complex pathophysiology. 2023;4(2):134–59.
Courties A, Berenbaum F, Sellam JJJBS. The phenotypic approach to osteoarthritis: a look at metabolic syndrome-associated osteoarthritis. 2019;86(6):725–730.
Zhuo Q, et al. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol. 2012;8(12):729–37.
Askari A, et al. Relationship between metabolic syndrome and osteoarthritis: the Fasa Osteoarthritis Study. Diabetes Metab Syndr. 2017;11:S827–32.
He Y, et al. Pathogenesis of osteoarthritis: risk factors, regulatory pathways in chondrocytes, and experimental models. 2020;9(8):194.
Al Khatib F, et al. Biomechanical characteristics of the knee joint during gait in obese versus normal subjects. 2022;19(2):989.
Coggon D, et al. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord. 2001;25(5):622–7.
Sun AR, et al. Cartilage tissue engineering for obesity-induced osteoarthritis: physiology, challenges, and future prospects. Journal of Orthopaedic Translation. 2021;26:3–15.
Chen L, et al. Pathogenesis and clinical management of obesity-related knee osteoarthritis: impact of mechanical loading. Journal of Orthopaedic Translation. 2020;24:66–75.
Zhang X, et al. Relationship between knee muscle strength and fat/muscle mass in elderly women with knee osteoarthritis based on dual-energy X-ray absorptiometry. 2020;17(2):573.
Yanoshita M, et al. Cyclic tensile strain upregulates pro-inflammatory cytokine expression via FAK-MAPK signaling in chondrocytes. Inflammation. 2018;41(5):1621–30.
Hirose N, et al. Protective effects of cilengitide on inflammation in chondrocytes under excessive mechanical stress. 2020;44(4):966–74.
Hikida M, Nakajima M, Nakata K. Cyclic compressive mechanical loading on threedimensional cultured tissue of human chondrocytes synergistically upregulates MMP3 gene expression with IL-1β. J Osaka Dent Univ. 2021;55(1):91–8.
Zhang H, et al. Mechanical overloading promotes chondrocyte senescence and osteoarthritis development through downregulating FBXW7. 2022;81(5):676–686.
Roemhildt ML, et al. Chronic in vivo load alteration induces degenerative changes in the rat tibiofemoral joint. Osteoarthr Cartil. 2013;21(2):346–57.
• Pragasam SSJ, Venkatesan V. Metabolic syndrome predisposes to osteoarthritis: lessons from model system. CARTILAGE. 2020;1947603520980161. The incidence of knee OA associated with metabolic syndrome in an obese mutant rodent model confirms the role played by various components of MetS in promoting the incidence of OA
Zhu, J., et al., Instability and excessive mechanical loading mediate subchondral bone changes to induce osteoarthritis. 2020, 2020;8(6):350.
Kovács B, Vajda E, Nagy EE. Regulatory effects and interactions of the Wnt and OPG-RANKL-RANK signaling at the bone-cartilage interface in osteoarthritis. 2019;20(18):4653.
Karimi MT. and F. Hemati, Knee joint osteoarthritis in obese subjects, effects of diet and exercise on knee-joint loading: a review of literature. 2022;33(4):376–83.
Gløersen M, et al. Associations of body mass index with pain and the mediating role of inflammatory biomarkers in people with hand osteoarthritis. 2022;74(5):810–7.
Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50.
Lu J, et al. Adipose tissue-resident immune cells in obesity and type 2 diabetes. 2019;10(1173).
Castoldi A, et al. The macrophage switch in obesity development. Front Immunol. 2015;6:637.
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–56.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Investig. 2007;117(1):175–84.
Liu S, et al. Cartilage tissue engineering: from proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments (review). Mol Med Rep. 2022;25(3):99.
Purdy JC, Shatzel JJ. The hematologic consequences of obesity. 2021;106(3):306–19.
Sun AR, et al. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS ONE. 2017;12(8): e0183693.
Zhang H, Cai D, Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020;28(5):555–61. Activated macrophages generate pro-inflammatory mediators, as well as multiple tissue-degrading enzymes that escalate the inflammatory milieu and contribute to the destruction of cartilage and bone.
Jenei-Lanzl Z, Meurer A, Zaucke F. Interleukin-1β signaling in osteoarthritis – chondrocytes in focus. Cell Signal. 2019;53:212–23.
Molnar V, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. 2021;22(17):9208.
Zhang X, et al. Telmisartan mitigates TNF-α-induced type II collagen reduction by upregulating SOX-9. ACS Omega. 2021;6(17):11756–61.
Xu Z, et al. Agonism of GPR120 prevented IL-1β-induced reduction of extracellular matrix through SOX-9. Aging (Albany NY). 2020;12(12):12074–85.
Wiegertjes R, van de Loo FAJ, Blaney Davidson ENA. roadmap to target interleukin-6 in osteoarthritis. Rheumatology (Oxford). 2020;59(10):2681–2694.
Na HS, et al. Interleukin-1-interleukin-17 signaling axis induces cartilage destruction and promotes experimental osteoarthritis. 2020;11.
Kang S, et al. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007–23.
• Mimpen JY, et al. Interleukin-17A causes osteoarthritis-like transcriptional changes in human osteoarthritis-derived chondrocytes and synovial fibroblasts in vitro. 2021;12. IL-17 cytokines activated multiple catabolic pathways in knee OA patients making it a potential biomarker and a clincal target in OA
Zhu X, et al. Phenotypic alteration of macrophages during osteoarthritis: a systematic review. Arthritis Res Ther. 2021;23(1):110.
Liu B, et al. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp Ther Med. 2018;16(6):5009–14.
**e J, et al. Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee. 2019;46:36–44.
Wang W, et al. Targeting macrophage polarization as a promising therapeutic strategy for the treatment of osteoarthritis. Int Immunopharmacol. 2023;116: 109790.
Gu X, et al. Adipose tissue adipokines and lipokines: functions and regulatory mechanism in skeletal muscle development and homeostasis. Metabolism. 2023;139: 155379.
**e C, Chen Q. Adipokines: new therapeutic target for osteoarthritis? Curr Rheumatol Rep. 2019;21(12):71.
Yan M, et al. The role of leptin in osteoarthritis. Medicine (Baltimore). 2018;97(14): e0257.
Lambova SN, et al. Serum leptin and resistin levels in knee osteoarthritis—clinical and radiologic links: towards precise definition of metabolic type knee osteoarthritis. 2021;9(8):1019.
Min S, et al. Serum levels of leptin, osteopontin, and sclerostin in patients with and without knee osteoarthritis. Clin Rheumatol. 2021;40(1):287–94.
Kroon FPB, et al. The role of leptin and adiponectin as mediators in the relationship between adiposity and hand and knee osteoarthritis. Osteoarthritis Cartilage. 2019;27(12):1761–7.
Zhu J, et al. Association of serum levels of inflammatory markers and adipokines with joint symptoms and structures in participants with knee osteoarthritis. Rheumatology. 2021;61(3):1044–52. A complex interplay between the metabolic components and inflammatory markers have been found to be associated with joint symptoms and strucutral deformities in knee OA.
Dumond H, et al. Evidence for a key role of leptin in osteoarthritis. 2003;48(11):3118–29.
Gao Y-H, et al. An update on the association between metabolic syndrome and osteoarthritis and on the potential role of leptin in osteoarthritis. Cytokine. 2020;129: 155043.
Simopoulou T, et al. Differential expression of leptin and leptin’s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. 2007;15(8):872–883.
Ouchi N, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97.
Griffin TM, et al. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60(10):2935–44.
Presle N, et al. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthr Cartil. 2006;14(7):690–5.
Lee YH, Song GG. Association between circulating adiponectin levels and osteoarthritis: a meta-analysis. jrd. 2018;25(4):231–238.
Tang Q, et al. Association of osteoarthritis and circulating adiponectin levels: a systematic review and meta-analysis. Lipids Health Dis. 2018;17(1):189.
Laurberg TB, et al. Plasma adiponectin in patients with active, early, and chronic rheumatoid arthritis who are steroid- and disease-modifying antirheumatic drug-naive compared with patients with osteoarthritis and controls. 2009;36(9):1885–1891.
Xu H, et al. Increased adiponectin levels are associated with higher radiographic scores in the knee joint, but not in the hand joint. Sci Rep. 2021;11(1):1842.
Francin PJ, et al. Association between adiponectin and cartilage degradation in human osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):519–26.
Wu J, et al. Associations between circulating adipokines and bone mineral density in patients with knee osteoarthritis: a cross-sectional study. BMC Musculoskelet Disord. 2018;19(1):16.
Orellana C, et al. Synovial adiponectin was more associated with clinical severity than synovial leptin in women with knee osteoarthritis. Cartilage. 2021;13(1_suppl):1675s-1683s.
Samal B, et al. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14(2):1431–7.
Azamar-Llamas D, et al. Adipokine contribution to the pathogenesis of osteoarthritis. 2017;2017.
Han DF, et al. An update on the emerging role of visfatin in the pathogenesis of osteoarthritis and pharmacological intervention. Evid Based Complement Alternat Med. 2020;2020:8303570.
Conde J, et al. Differential expression of adipokines in infrapatellar fat pad (IPFP) and synovium of osteoarthritis patients and healthy individuals. Ann Rheum Dis. 2014;73(3):631–3.
Duan Y, et al. Increased synovial fluid visfatin is positively linked to cartilage degradation biomarkers in osteoarthritis. Rheumatol Int. 2012;32(4):985–90.
Junker S, et al. Expression of adipokines in osteoarthritis osteophytes and their effect on osteoblasts. Matrix Biol. 2017;62:75–91.
Moschen AR, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178(3):1748–58.
Zhao C-W, et al. An update on the emerging role of resistin on the pathogenesis of osteoarthritis. Mediators Inflamm. 2019;2019:1532164.
Lambova SN, et al. Serum leptin and resistin levels in knee osteoarthritis-clinical and radiologic links: towards precise definition of metabolic type knee osteoarthritis. Biomedicines. 2021;9(8).
Alissa EM, Alzughaibi LS, Marzouki ZM. Relationship between serum resistin, body fat and inflammatory markers in females with clinical knee osteoarthritis. Knee. 2020;27(1):45–50.
Lim DH, Choi S. High synovial fluid resistin levels are associated with radiographic severity in female patients with knee osteoarthritis. Osteoarthritis Cartilage. 2020;28:S195–6.
Han W, et al. Higher serum levels of resistin are associated with knee synovitis and structural abnormalities in patients with symptomatic knee osteoarthritis. J Am Med Dir Assoc. 2019;20(10):1242–6.
Iannone F, Lapadula G. Chemerin/ChemR23 pathway: a system beyond chemokines. Arthritis Res Ther. 2011;13(2):104.
Huang K, et al. Association of chemerin levels in synovial fluid with the severity of knee osteoarthritis. Biomarkers. 2012;17(1):16–20.
Berg V, et al. Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin. Arthritis Res Ther. 2010;12:R228.
Carrión M, et al. The adipokine network in rheumatic joint diseases. 2019;20(17):4091.
Gupta K, et al. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56(10):3326–35.
Puzio I, et al. Role of nesfatin-1 in the metabolism of skeletal tissues. 2018;74(5):290–94.
Zhang Y, et al. Serum and synovial fluid nesfatin-1 concentration is associated with radiographic severity of knee osteoarthritis. Medical science monitor : international medical journal of experimental and clinical research. 2015;21:1078–82.
Jiang L, et al. Increased serum levels and chondrocyte expression of nesfatin-1 in patients with osteoarthritis and its relation with BMI, hsCRP, and IL-18. Mediators Inflamm. 2013;2013: 631251.
Jiang L, et al. Nesfatin-1 suppresses interleukin-1β-induced inflammation, apoptosis, and cartilage matrix destruction in chondrocytes and ameliorates osteoarthritis in rats. Aging (Albany NY). 2020;12(2):1760–77.
Hu P-F, et al. Increased apelin serum levels and expression in human chondrocytes in osteoarthritic patients. Int Orthop. 2011;35(9):1421–6.
Chang TK, et al. Apelin enhances IL-1β expression in human synovial fibroblasts by inhibiting miR-144-3p through the PI3K and ERK pathways. Aging (Albany NY). 2020;12(10):9224–39.
Hu P-F, et al. Apelin plays a catabolic role on articular cartilage: in vivo and in vitro studies. Int J Mol Med. 2010;26(3):357–63.
Wang Y-H, et al. Apelin affects the progression of osteoarthritis by regulating VEGF-dependent angiogenesis and miR-150–5p expression in human synovial fibroblasts. 2020;9(3):594.
Conde J, et al. Identification of novel adipokines in the joint. Differential expression in healthy and osteoarthritis tissues. PLOS ONE. 2015;10(4):e0123601.
Geurts J, et al. A novel Saa3-promoter reporter distinguishes inflammatory subtypes in experimental arthritis and human synovial fibroblasts. 2011;70(7):1311–1319.
Sobieh BH, et al. Potential emerging roles of the novel adipokines adipolin/CTRP12 and meteorin-like/METRNL in obesity-osteoarthritis interplay. Cytokine. 2021;138: 155368.
Scotece M, et al. Novel adipokine associated with OA: retinol binding protein 4 (RBP4) is produced by cartilage and is correlated with MMPs in osteoarthritis patients. Inflamm Res. 2020;69(4):415–21.
Zhang C, et al. FABP4 as a biomarker for knee osteoarthritis. Biomark Med. 2018;12(2):107–18.
Valverde-Franco G, et al. High in vivo levels of adipsin lead to increased knee tissue degradation in osteoarthritis: data from humans and animal models. Rheumatology. 2018;57(10):1851–60.
Paré F, et al. In vivo protective effect of adipsin-deficiency on spontaneous knee osteoarthritis in aging mice. Aging (Albany NY). 2020;12(3):2880–96.
Feng D, et al. Progranulin modulates cartilage-specific gene expression via sirtuin 1-mediated deacetylation of the transcription factors SOX9 and P65. J Biol Chem. 2020;295(39):13640–50.
He H, et al. Vaspin regulated cartilage cholesterol metabolism through miR155/LXRα and participated in the occurrence of osteoarthritis in rats. Life Sci. 2021;269: 119096.
Santoro A, et al. SERPINE2 inhibits IL-1α-induced MMP-13 expression in human chondrocytes: involvement of ERK/NF-κB/AP-1 pathways. PLoS ONE. 2015;10(8): e0135979.
Li Z, et al. Omentin-1 promotes mitochondrial biogenesis via PGC1α-AMPK pathway in chondrocytes. Arch Physiol Biochem. 2020;1–7.
Sanja Klobučar M, et al. Dyslipidemia: current perspectives and implications for clinical practice. In: Management of dyslipidemia, Wilbert SA. Editor. 2021;IntechOpen: Rijeka.Ch.1.
Cho BW, et al. Cross-sectional association between hypercholesterolemia and knee pain in the elderly with radiographic knee osteoarthritis: data from the Korean National Health and Nutritional Examination Survey. 2021;10(5):933.
Farnaghi S, et al. Protective effects of mitochondria-targeted antioxidants and statins on cholesterol-induced osteoarthritis. Faseb j. 2017;31(1):356–67.
Tsezou A, et al. Impaired expression of genes regulating cholesterol efflux in human osteoarthritic chondrocytes. J Orthop Res. 2010;28(8):1033–9.
Papathanasiou I, Anastasopoulou L, Tsezou A. Cholesterol metabolism related genes in osteoarthritis. Bone. 2021;152: 116076.
Zheng L, et al. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev. 2021;66: 101249.
Choi W-S, et al. The CH25H–CYP7B1–RORα axis of cholesterol metabolism regulates osteoarthritis. Nature. 2019;566(7743):254–8.
Cao C, et al. Cholesterol-induced LRP3 downregulation promotes cartilage degeneration in osteoarthritis by targeting Syndecan-4. Nat Commun. 2022;13(1):7139.
de Munter W, et al. Cholesterol accumulation caused by low density lipoprotein receptor deficiency or a cholesterol-rich diet results in ectopic bone formation during experimental osteoarthritis. Arthritis Res Ther. 2013;15(6):R178.
Davies-Tuck ML, et al. Total cholesterol and triglycerides are associated with the development of new bone marrow lesions in asymptomatic middle-aged women - a prospective cohort study. Arthritis Res Ther. 2009;11(6):R181.
Triantaphyllidou IE, et al. Perturbations in the HDL metabolic pathway predispose to the development of osteoarthritis in mice following long-term exposure to western-type diet. Osteoarthritis Cartilage. 2013;21(2):322–30.
Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94.
Bostan M, et al. Effects of synovial fluid on the respiratory burst of granulocytes in rheumatoid arthritis. J Cell Mol Med. 2001;5(2):188–94.
Nishimura S, et al. Oxidized low-density lipoprotein (ox-LDL) binding to lectin-like ox-LDL receptor-1 (LOX-1) in cultured bovine articular chondrocytes increases production of intracellular reactive oxygen species (ROS) resulting in the activation of NF-kappaB. Osteoarthritis Cartilage. 2004;12(7):568–76.
Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–66.
Kanata S, et al. Oxidized LDL binding to LOX-1 upregulates VEGF expression in cultured bovine chondrocytes through activation of PPAR-gamma. Biochem Biophys Res Commun. 2006;348(3):1003–10.
Simopoulou T, Malizos KN, Tsezou A. Lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) expression in human articular chondrocytes. Clin Exp Rheumatol. 2007;25(4):605–12.
Harasymowicz NS, et al. Physiologic and pathologic effects of dietary free fatty acids on cells of the joint. Ann N Y Acad Sci. 2019;1440(1):36–53.
Shin Y, et al. Low-density lipoprotein receptor-related protein 5 governs Wnt-mediated osteoarthritic cartilage destruction. Arthritis Res Ther. 2014;16(1):R37.
Hashimoto K, Akagi M. The role of oxidation of low-density lipids in pathogenesis of osteoarthritis: a narrative review. J Int Med Res. 2020;48(6):300060520931609.
Wu X, et al. The metabolic landscape in osteoarthritis. Aging Dis. 2022;13(4):1166–82.
Tsai Y-W, et al. Palmitoleic acid ameliorates palmitic acid-induced proinflammation in J774A.1 macrophages via TLR4-dependent and TNF-α-independent signallings. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2021;169:102270.
Sekar S, et al. Saturated fatty acids promote chondrocyte matrix remodeling through reprogramming of autophagy pathways. Nutrition. 2018;54:144–52.
Tan L, et al. Dietary saturated fatty acid palmitate promotes cartilage lesions and activates the unfolded protein response pathway in mouse knee joints. PLoS ONE. 2021;16(2): e0247237.
Veronese N, et al. Type 2 diabetes mellitus and osteoarthritis. Semin Arthritis Rheum. 2019;49(1):9–19.
Sellam J, Berenbaum F. Is osteoarthritis a metabolic disease? Joint Bone Spine. 2013;80(6):568–73.
Louati K, et al. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open. 2015;1(1): e000077.
Ashrafizadeh H, Ashrafizadeh M, Oroojan AA. Type 2 diabetes mellitus and osteoarthritis: the role of glucose transporters. Clinical Reviews in Bone and Mineral Metabolism. 2020;18(1):1–17.
Juybari KB, Hosseinzadeh A, Sharifi AM. Protective effects of atorvastatin against high glucose-induced nuclear factor-κB activation in cultured C28I2 chondrocytes. J Recept Signal Transduction. 2019;39(1):1–8.
Njoto I, et al. Chondrocyte intracellular matrix strain fields of articular cartilage surface in hyperglycemia model of rat: cellular morphological study. Med Arch. 2018;72(5):348–51.
Doré S, et al. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation. Possible role of IGF-1-binding proteins. Arthritis Rheum. 1994;37(2):253–63.
Bolduc JA, et al. Reactive oxygen species, aging and articular cartilage homeostasis. 2019;132:73–82.
Zhuang C, et al. Oxidative stress induces chondrocyte apoptosis through caspase-dependent and caspase-independent mitochondrial pathways and the antioxidant mechanism of Angelica sinensis polysaccharide. 2020;2020.
Kan S, et al. Role of mitochondria in physiology of chondrocytes and diseases of osteoarthritis and rheumatoid arthritis. 2021;13(2_suppl):1102S-1121S.
Moshtagh PR, et al. Effects of non-enzymatic glycation on the micro- and nano-mechanics of articular cartilage. J Mech Behav Biomed Mater. 2018;77:551–6.
Suzuki A, Yabu A, Nakamura H. Advanced glycation end products in musculoskeletal system and disorders. Methods. 2022;203:179–86.
Yang Q, et al. Advanced glycation end products induced mitochondrial dysfunction of chondrocytes through repression of AMPKα-SIRT1-PGC-1α pathway. Pharmacology. 2022;107(5–6):298–307.
Li Q, et al. Hyperglycemia-induced accumulation of advanced glycosylation end products in fibroblast-like synoviocytes promotes knee osteoarthritis. Exp Mol Med. 2021;53(11):1735–47.
Wang HJ, et al. Diabetes mellitus accelerates the progression of osteoarthritis in streptozotocin-induced diabetic mice by deteriorating bone microarchitecture, bone mineral composition, and bone strength of subchondral bone. Ann Transl Med. 2021;9(9):768.
Davies-Tuck ML, et al. Increased fasting serum glucose concentration is associated with adverse knee structural changes in adults with no knee symptoms and diabetes. Maturitas. 2012;72(4):373–8.
Bradley D. The intriguing intersection of type 2 diabetes, obesity-related insulin resistance, and osteoarthritis. J Clin Endocrinol Metab. 2021;106(5):e2370–2.
Sakhrani N, et al. Toward development of a diabetic synovium culture model. Front Bioeng Biotechnol. 2022;10: 825046.
Griffin TM, Huffman KM. Editorial: Insulin resistance: releasing the brakes on synovial inflammation and osteoarthritis? Arthritis Rheumatol. 2016;68(6):1330–3.
Qiao L, Li Y, Sun S. Insulin exacerbates inflammation in fibroblast-like synoviocytes. Inflammation. 2020;43(3):916–36.
Ribeiro M, et al. Insulin decreases autophagy and leads to cartilage degradation. Osteoarthritis Cartilage. 2016;24(4):731–9.
Herrero-Beaumont G, et al. Targeting chronic innate inflammatory pathways, the main road to prevention of osteoarthritis progression. Biochem Pharmacol. 2019;165:24–32.
Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446(7139):1017–22.
Tardio L, et al. O-linked N-acetylglucosamine (O-GlcNAc) protein modification is increased in the cartilage of patients with knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):259–63.
Kokubo Y, Iwashima Y. Higher blood pressure as a risk factor for diseases other than stroke and ischemic heart disease. Hypertension. 2015;66(2):254–9.
Ching K, et al. Hypertension meets osteoarthritis — revisiting the vascular aetiology hypothesis. Nat Rev Rheumatol. 2021;17(9):533–49.
Niu J, et al. Metabolic syndrome, its components, and knee osteoarthritis: the Framingham Osteoarthritis Study. Arthritis Rheumatol. 2017;69(6):1194–203.
Khamidov O, et al. The role of vascular pathology in the development and progression of deforming osteoarthritis of the joints of the lower extremities (Literature review). 2021:214–225.
Findlay DM. Vascular pathology and osteoarthritis. Rheumatology (Oxford). 2007;46(12):1763–8.
Shi X, Schlenk EAJPMN. Association of hypertension with knee pain severity among people with knee osteoarthritis. 2022;23(2):135–141.
Zhang YM, Wang J, Liu XG. Association between hypertension and risk of knee osteoarthritis: a meta-analysis of observational studies. Medicine (Baltimore). 2017;96(32): e7584.
Lo K, et al. Association between hypertension and osteoarthritis: a systematic review and meta-analysis of observational studies. Journal of Orthopaedic Translation. 2022;32:12–20. A significant relationship between hypertension and structural damages of knee OA points to a plausible vascular etiology of OA.
Onur T, et al. Characterisation of osteoarthritis in a small animal model of type 2 diabetes mellitus. Bone Joint Res. 2014;3(6):203–11.
Fu Y, et al. Effects of leptin and body weight on inflammation and knee osteoarthritis phenotypes in female rats. n/a;(n/a):e10754.
Deng C, et al. Eplerenone treatment alleviates the development of joint lesions in a new rat model of spontaneous metabolic-associated osteoarthritis. Ann Rheum Dis. 2018;77(2):315–6.
Siriarchavatana P, et al. The preventive effects of greenshell mussel (Perna canaliculus) on early-stage metabolic osteoarthritis in rats with diet-induced obesity. 2019;11(7):1601.
Radakovich LB, et al. Calorie restriction with regular chow, but not a high-fat diet, delays onset of spontaneous osteoarthritis in the Hartley guinea pig model. Arthritis Res Ther. 2019;21(1):145.
Rui F, et al. Undenatured type II collagen prevents and treats osteoarthritis and motor function degradation in T2DM patients and db/db mice. Food Funct. 2021;12(10):4373–91.
Georgiev T, Angelov AK. Modifiable risk factors in knee osteoarthritis: treatment implications. Rheumatol Int. 2019;39(7):1145–57.
Salis Z, et al. Weight loss is associated with reduced risk of knee and hip replacement: a survival analysis using Osteoarthritis Initiative data. Int J Obes. 2022;46(4):874–84.
Raposo F, Ramos M. and A. Lúcia Cruz, Effects of exercise on knee osteoarthritis: a systematic review. 2021;19(4):399–435.
Xu C, et al. Dietary patterns and risk of develo** knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2021;29(6):834–40.
Song, Y, Wu Z, Zhao P. The effects of metformin in the treatment of osteoarthritis: Current perspectives. 2022;13.
Liu X, et al. Metformin attenuates high-fat diet induced metabolic syndrome related osteoarthritis through inhibition of prostaglandins. Front Cell Dev Biol. 2023;11:1184524.
Li D, et al. Metformin attenuates osteoarthritis by targeting chondrocytes, synovial macrophages and adipocytes. Rheumatology. 2022;62(4):1652–61.
Maderitz RLJLJoMS. Easing the burden: the value of lifestyle modifications for management of knee osteoarthritis in patients with metabolic syndrome. 2023;5(1):102.
Park D, et al. Association of general and central obesity, and their changes with risk of knee osteoarthritis: a nationwide population-based cohort study. Sci Rep. 2023;13(1):3796. General obesity and central obesity are proven risk factors for OA.
Chen L, et al. The burden of end-stage osteoarthritis in Australia: a population-based study on the incidence of total knee replacement attributable to overweight/obesity. Osteoarthritis Cartilage. 2022;30(9):1254–62.
Raud B, et al. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci Rep. 2020;10(1):3601.
Misra D, et al. Risk of knee osteoarthritis with obesity, sarcopenic obesity, and sarcopenia. Arthritis Rheumatol. 2019;71(2):232–7.
Hussain SM, et al. Relationship of weight and obesity with the risk of knee and hip arthroplasty for osteoarthritis across different levels of physical performance: a prospective cohort study. Scand J Rheumatol. 2019;48(1):64–71.
Reyes C, et al. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatol. 2016;68(8):1869–75.
Lee S, et al. Obesity, metabolic abnormality, and knee osteoarthritis: a cross-sectional study in Korean women. Mod Rheumatol. 2015;25(2):292–7.
Apold H, et al. Weight gain and the risk of knee replacement due to primary osteoarthritis: a population based, prospective cohort study of 225,908 individuals. Osteoarthritis Cartilage. 2014;22(5):652–8.
Mork PJ, Holtermann A, Nilsen TI. Effect of body mass index and physical exercise on risk of knee and hip osteoarthritis: longitudinal data from the Norwegian HUNT Study. J Epidemiol Community Health. 2012;66(8):678–83.
Holliday KL, et al. Lifetime body mass index, other anthropometric measures of obesity and risk of knee or hip osteoarthritis in the GOAL case-control study. Osteoarthritis Cartilage. 2011;19(1):37–43.
Yoshimura N. Epidemiology of osteoarthritis in Japan: the ROAD study. Clin Calcium. 2011;21(6):821–5.
Toivanen AT, et al. Obesity, physically demanding work and traumatic knee injury are major risk factors for knee osteoarthritis—a population-based study with a follow-up of 22 years. Rheumatology (Oxford). 2010;49(2):308–14.
Lohmander LS, et al. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis. 2009;68(4):490–6.
Grotle M, et al. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132.
Reijman M, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66(2):158–62.
Karlson EW, et al. Total hip replacement due to osteoarthritis: the importance of age, obesity, and other modifiable risk factors. Am J Med. 2003;114(2):93–8.
Gelber AC, et al. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Access the “Journal Club” discussion of this paper at http://www.elsevier.com/locate/ajmselect. Am J Med. 1999;107(6):542–548.
Shiozaki H, et al. Obesity and osteoarthritis of the knee in women: results from the Matsudai Knee Osteoarthritis survey. Knee. 1999;6(3):189–92.
Felson DT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40(4):728–33.
Hart DJ, Spector TD. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol. 1993;20(2):331–5.
Felson DT, et al. Obesity and knee osteoarthritis. The Framingham Study Ann Intern Med. 1988;109(1):18–24.
Hartz AJ, et al. The association of obesity with joint pain and osteoarthritis in the HANES data. J Chronic Dis. 1986;39(4):311–9.
Otero M, et al. Signalling pathway involved in nitric oxide synthase type II activation in chondrocytes: synergistic effect of leptin with interleukin-1. Arthritis Res Ther. 2005;7(3):R581.
Simopoulou T, et al. Differential expression of leptin and leptin’s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthritis Cartilage. 2007;15(8):872–83.
Lago R, et al. A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthritis Cartilage. 2008;16(9):1101–9.
Vuolteenaho K, et al. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage—mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators Inflamm. 2009;2009:345838–345838.
Bao J-P, et al. Leptin plays a catabolic role on articular cartilage. Mol Biol Rep. 2010;37(7):3265–72.
Mutabaruka M-S, et al. Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Res Ther. 2010;12(1):R20.
Conde J, et al. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS ONE. 2012;7(12): e52533.
Hui W, et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. 2012;71(3):455–62.
Yang W-H, et al. Leptin induces IL-6 expression through OBRl receptor signaling pathway in human synovial fibroblasts. PLoS ONE. 2013;8(9): e75551.
Yaykasli KO, et al, Leptin induces ADAMTS-4, ADAMTS-5, and ADAMTS-9 genes expression by mitogen-activated protein kinases and NF-ĸB signaling pathways in human chondrocytes. 2015;39(1):104–112.
Koskinen A, et al. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin Exp Rheumatol. 2011;29(1):57–64.
Scotece M, et al. Adipokines induce pro-inflammatory factors in activated Cd4+ T cells from osteoarthritis patient. 2017;35(6):1299–1303.
Zhang ZM, et al. Leptin induces the apoptosis of chondrocytes in an in vitro model of osteoarthritis via the JAK2-STAT3 signaling pathway. Mol Med Rep. 2016;13(4):3684–90.
Huang ZM, et al. Leptin promotes apoptosis and inhibits autophagy of chondrocytes through upregulating lysyl oxidase-like 3 during osteoarthritis pathogenesis. Osteoarthritis Cartilage. 2016;24(7):1246–53.
Wang W, et al. Effects of the leptin-mediated MAPK/ERK signaling pathway on collagen II expression in knee cartilage of newborn male mice from obese maternal offspring. 2022;12(3):477.
Zhang S, et al. Effects of leptin on differentiation and proliferation of chondrocytes. Journal of Hard Tissue Biology. 2019;28(1):51–6.
Conde J, et al. E74-like factor (ELF3) and leptin, a novel loop between obesity and inflammation perpetuating a pro-catabolic state in cartilage. Cell Physiol Biochem. 2018;45(6):2401–10.
Su Y-P, et al. Leptin induces MMP1/13 and ADAMTS 4 expressions through bone morphogenetic protein-2 autocrine effect in human chondrocytes. J Cell Biochem. 2018;119(4):3716–24.
**ong H, et al. Elevated leptin levels in temporomandibular joint osteoarthritis promote proinflammatory cytokine IL-6 expression in synovial fibroblasts. 2019;48(3):251–259.
Zhao X, et al. Activation of the leptin pathway by high expression of the long form of the leptin receptor (Ob-Rb) accelerates chondrocyte senescence in osteoarthritis. 2019;8(9):425–436.
Mourmoura E, et al. Leptin-depended NLRP3 inflammasome activation in osteoarthritic chondrocytes is mediated by ROS. Mech Ageing Dev. 2022;208: 111730.
Primrose JGB, et al. Concentration-dependent effects of leptin on osteoarthritis-associated changes in phenotype of human chondrocytes. Connect Tissue Res. 2023;1–12.
Liang J, et al. Leptin-mediated cytoskeletal remodeling in chondrocytes occurs via the RhoA/ROCK pathway. J Orthop Res. 2011;29(3):369–74.
Gómez R, et al. Adiponectin and leptin increase IL-8 production in human chondrocytes. 2011;70(11):2052–2054.
Jiang M, et al. Leptin induced TLR4 expression via the JAK2-STAT3 pathway in obesity-related osteoarthritis. Oxid Med Cell Longev. 2021;2021:7385160.
Strebkova E, et al. The role of leptin in the metabolic phenotype of osteoarthritis. Osteoarthritis Cartilage. 2021;29:S158. Leptin is an aggravating predcitor of metabolic syndrome associated OA with higher leptin levels found in more advanced stages of OA making it a potential therapeutic target.
Tang CH, et al. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J Immunol. 2007;179(8):5483–92.
Kang EH, et al. Adiponectin is a potential catabolic mediator in osteoarthritis cartilage. Arthritis Res Ther. 2010;12(6):R231.
Tong K-M, et al. Adiponectin increases MMP-3 expression in human chondrocytes through adipor1 signaling pathway. 2011;112(5):1431–1440.
Zuo W, et al. Adiponectin receptor 1 mediates the difference in adiponectin-induced prostaglandin E2 production in rheumatoid arthritis and osteoarthritis synovial fibroblasts. 2011 Chinese Medical Journals Publishing House Co., Ltd. 42 Dongsi **dajie. 3919–3924.
Chen H-T, et al. Adiponectin enhances intercellular adhesion molecule-1 expression and promotes monocyte adhesion in human synovial fibroblasts. PLoS ONE. 2014;9(3): e92741.
Udomsinprasert W, et al. Decreased serum adiponectin reflects low vitamin D, high interleukin 6, and poor physical performance in knee osteoarthritis. Arch Immunol Ther Exp. 2020;68(3):16.
Ali SA, et al. Adiponectin is a potential mediator of synovial fibrosis from early to late knee osteoarthritis. Osteoarthritis Cartilage. 2019;27:S479–80.
Harasymowicz NS, et al. Chondrocytes from osteoarthritic cartilage of obese patients show altered adiponectin receptors expression and response to adiponectin. 2021;39(11):2333–9.
Gosset M, et al. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: possible influence on osteoarthritis. Arthritis Rheum. 2008;58(5):1399–409.
Dvir-Ginzberg M, et al. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase*. J Biol Chem. 2008;283(52):36300–10.
McNulty AL, et al. The effects of adipokines on cartilage and meniscus catabolism. Connect Tissue Res. 2011;52(6):523–33.
Hong EH, et al. Nicotinamide phosphoribosyltransferase is essential for interleukin-1beta-mediated dedifferentiation of articular chondrocytes via SIRT1 and extracellular signal-regulated kinase (ERK) complex signaling. J Biol Chem. 2011;286(32):28619–31.
Yammani RR, Loeser RF. Extracellular nicotinamide phosphoribosyltransferase (NAMPT/visfatin) inhibits insulin-like growth factor-1 signaling and proteoglycan synthesis in human articular chondrocytes. Arthritis Res Ther. 2012;14(1):R23.
Chauffier K, et al. Induction of the chemokine IL-8/Kc by the articular cartilage: possible influence on osteoarthritis. Joint Bone Spine. 2012;79(6):604–9.
Pecchi E, et al. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis pain. Arthritis Res Ther. 2014;16(1):R16.
Laiguillon M-C, et al. Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis. Arthritis Res Ther. 2014;16(1):R38.
Yang S, et al. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2α, is an essential catabolic regulator of osteoarthritis. Ann Rheum Dis. 2015;74(3):595–602.
Oh H, et al. Reciprocal regulation by hypoxia-inducible factor-2α and the NAMPT-NAD+-SIRT axis in articular chondrocytes is involved in osteoarthritis. Osteoarthritis Cartilage. 2015;23(12):2288–96.
Won Y, et al. Pleiotropic roles of metallothioneins as regulators of chondrocyte apoptosis and catabolic and anabolic pathways during osteoarthritis pathogenesis. 2016;75(11):2045–52.
Cheleschi S, et al. Exploring the crosstalk between hydrostatic pressure and adipokines: an in vitro study on human osteoarthritic chondrocytes. 2021;22(5):2745.
Wu M-H, et al. Visfatin promotes IL-6 and TNF-α production in human synovial fibroblasts by repressing miR-199a-5p through ERK, p38 and JNK signaling pathways. 2018;19(1):190.
Cheleschi S, et al. MicroRNA mediate visfatin and resistin induction of oxidative stress in human osteoarthritic synovial fibroblasts via NF-κB pathway. 2019;20(20):5200.
Law Y-Y, et al. Visfatin increases ICAM-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by reducing miR-320a expression. Aging. 2020;12(18):18635–48.
Chang, S-F, et al. Effects of visfatin on intracellular mechanics and catabolism in human primary chondrocytes through glycogen synthase kinase 3β inactivation. 2021;22(15):8107.
Cheleschi S, et al. MicroRNA-34a and microRNA-181a mediate visfatin-induced apoptosis and oxidative stress via NF-κB pathway in human osteoarthritic chondrocytes. 2019;8(8):874.
Philp AM, et al. eNAMPT is localised to areas of cartilage damage in patients with hip osteoarthritis and promotes cartilage catabolism and inflammation. 2021;22(13):6719.
Tsai C-H, et al. Visfatin increases VEGF-dependent angiogenesis of endothelial progenitor cells during osteoarthritis progression. 2020;9(5):1315.
Silswal N, et al. Human resistin stimulates the pro-inflammatory cytokines TNF-α and IL-12 in macrophages by NF-κB-dependent pathway. Biochem Biophys Res Commun. 2005;334(4):1092–101.
Lee JH, et al. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthritis Cartilage. 2009;17(5):613–20.
Zhang Z, et al. Resistin induces expression of proinflammatory cytokines and chemokines in human articular chondrocytes via transcription and messenger RNA stabilization. Arthritis Rheum. 2010;62(7):1993–2003.
Koskinen A, et al. Resistin as a factor in osteoarthritis: synovial fluid resistin concentrations correlate positively with interleukin 6 and matrix metalloproteinases MMP-1 and MMP-3. Scand J Rheumatol. 2014;43(3):249–53.
Zhang Z, et al. Resistin stimulates expression of chemokine genes in chondrocytes via combinatorial regulation of C/EBPβ and NF-κB. 2014;15(10):17242–55.
Chen W-C, et al. Resistin enhances VCAM-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by inhibiting MiR-381 expression through the PKC, p38, and JNK signaling pathways. 2020;9(6):1369.
Chen WC, et al. Resistin enhances IL-1β and TNF-α expression in human osteoarthritis synovial fibroblasts by inhibiting miR-149 expression via the MEK and ERK pathways. Faseb j. 2020;34(10):13671–84.
Eisinger K, et al. Chemerin induces CCL2 and TLR4 in synovial fibroblasts of patients with rheumatoid arthritis and osteoarthritis. Exp Mol Pathol. 2012;92(1):90–6.
Wang C, et al. Chemerin promotes MAPK/ERK activation to induce inflammatory factor production in rat synoviocytes. Exp Ther Med. 2022;24(5):684.
Conde J, et al. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. 2011;70(3):551–9.
Yu Chengshuai DG. Pang Shenning. Lao Shan, Chemerin, a pro-inflammatory adipokine, regulates chondrocyte proliferation and metabolism by increasing production of nitric oxide. 2021;25(2):258–63.
Villalvilla A, et al. The adipokine lipocalin-2 in the context of the osteoarthritic osteochondral junction. Sci Rep. 2016;6(1):29243.
Francisco V, et al. Adipokines and inflammation: is it a question of weight? 2018;175(10):1569–1579.
Jiang Z, et al. Whole-transcriptome sequence of degenerative meniscus cells unveiling diagnostic markers and therapeutic targets for osteoarthritis. Front Genet. 2021;12: 754421.
Shen F, et al. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77(3):388–99.
Scotece M, et al. NUCB2/nesfatin-1: a new adipokine expressed in human and murine chondrocytes with pro-inflammatory properties, an in vitro study. 2014;32(5):653–60.
Lee KT, et al. Nesfatin-1 facilitates IL-1β production in osteoarthritis synovial fibroblasts by suppressing miR-204-5p synthesis through the AP-1 and NF-κB pathways. Aging (Albany NY). 2021;13(18):22490–501.
Lu D, et al. Apelin alleviates meniscus endothelial cell apoptosis in osteoarthritis. Dis Markers. 2022;2022:3556372.
Takano S, et al. Vascular endothelial growth factor expression and their action in the synovial membranes of patients with painful knee osteoarthritis. BMC Musculoskelet Disord. 2018;19(1):204.
de Seny D, et al. Acute-phase serum amyloid A in osteoarthritis: regulatory mechanism and proinflammatory properties. PLoS ONE. 2013;8(6): e66769.
Zhang C, et al. FABP4 as a biomarker for knee osteoarthritis. 2018;12(2):107–118.
Zhao Y-P, et al. Progranulin protects against osteoarthritis through interacting with TNF-α and β-catenin signalling. 2015;74(12):2244–53.
Pan Y, et al. Progranulin regulation of autophagy contributes to its chondroprotective effect in osteoarthritis. Genes & Diseases. 2023;10(4):1582–95.
Guo F, et al. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010;62(7):2023–36.
Papers of particular interest, published recently, have been highlighted as: • Of importance
Bortoluzzi A, Furini F, Scirè CA. Osteoarthritis and its management - epidemiology, nutritional aspects and environmental factors. Autoimmun Rev. 2018;17(11):1097–104.
Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 2018;392(10159):1789–1858.
Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage. 2022;30(1):10–6.
Wang Y, et al. Knee osteoarthritis, potential mediators, and risk of all-cause mortality: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). 2021;73(4):566–73.
March L, et al. Osteoarthritis: A Serious Disease: Submitted to the US Food and Drug Administration. 2016.
Coaccioli S, et al. Osteoarthritis: new insight on its pathophysiology. 2022;11(20):6013.
Donell SJEOR. Subchondral bone remodelling in osteoarthritis. 2019;4(6):221.
Lee JY, et al. Association of leg muscle symmetry with knee osteoarthritis. Clin Rheumatol. 2019;38(12):3549–56.
Ozeki N, Koga H, Sekiya IJL. Degenerative meniscus in knee osteoarthritis: from pathology to treatment. 2022;12(4):603.
Schulze-Tanzil G. Intraarticular ligament degeneration is interrelated with cartilage and bone destruction in osteoarthritis. 2019;8(9):990.
Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30(2):184–95.
• Cui A, et al. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29. The global prevalence and incidence of knee OA increased with age and peaked at 70–79 years while increasing education attainment was negatively associated with the prevalence of knee OA.
Acknowledgements
The authors thank the Department of Health Research, Ministry of Health and Family Welfare, New Delhi for financial support to Samuel Joshua Pragasam Sampath through the DHR – Young Scientist Research Fellowship (DHR – YSS Grant No. YSS/2020/000185/PRCYSS).
Funding
Department of Health Research,Ministry of Health and Family Welfare,New Delhi,India,YSS/2020/000185/PRCYSS,Samuel Joshua Pragasam Sampath
Author information
Authors and Affiliations
Contributions
SJPS completed the review; KN, SG, and VV reviewed the content and approach of the manuscript, and critically evaluated the review.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sampath, S.J.P., Venkatesan, V., Ghosh, S. et al. Obesity, Metabolic Syndrome, and Osteoarthritis—An Updated Review. Curr Obes Rep 12, 308–331 (2023). https://doi.org/10.1007/s13679-023-00520-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-023-00520-5