Abstract
Five new toosendanin limonoids with highly oxidative furan ring walsurobustones A-D (1–4), and one new furan ring degraded limonoid walsurobustone E (5) together with one known compound toonapubesic acid B (6) were isolated from the leaves of Walsura robusta. Their structures were elucidated by NMR and MS data. Especially, the absolute configuration of toonapubesic acid B (6) was confirmed by X-ray diffraction study. Compounds 1–6 exhibited good cytotoxicity against the cancer cell lines HL-60, SMMC-7721, A-549, MCF-7, and SW480.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Limonoids, well known as tetranortriterpenoids, were widely found in the families of Meliaceae and Rutaceae, which have attracted great interests from the chemical and biological research communities [1, 2]. The genus Walsura (Meliaceae), comprising about 16 species, distributes in subtropical regions such as Southern China, India, and Indonesia [3]. In previous literature, kinds of natural products such as triterpenoids, phenols, and steroids have been identified in this genus. Some triterpenoids and phenols exhibited cell protection, antioxidation and antimalarial activities [4,5,6,7,8,9,10,11,12,13,14]. However, the chemical constituents from Walsura robusta and their activities study are few. We reported herein the isolation, characterization as well as cytotoxic activity of these highly oxidized limonoids and a plausible biosynthesis pathway of 5 and 6.
2 Results and discussion
The MeOH extract of Walsura yunnanensis was filtered and concentrated in vacuo to afford a residue, which was then defatted by petroleum ether and extracted with EtOAc. The EtOAc fraction was subjected to column chromatography on silica gel, ODS, HPLC, and Sephadex LH-20 to yield compounds 1–6 (detailed procedures see Extraction and Isolation part).
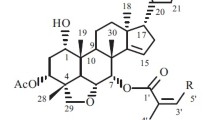
Walsurobustone A (1) was isolated as a white amorphous powder. Its molecular formula was determined to be C28H38O9 by the [M + Na]+ ion peak at m/z 541.2418 (calcd 541.2413 for C28H38O9Na) in the HRESIMS, with 16 mass units less than that of the known compound yunnanol A, isolated from the congener plant W. yunnanensis [15]. The 13C NMR data (Table 2) of 1 showed high similarity to those reported for yunnanol A, with the main difference is the absence of signals for the hydroxyl at C-11 (δH 1.81, 1H, m and 1.56, 1H, m; δC 19.2) in 1, while δH 5.03 (1H, m) and δC 67.0 in yunnanol A. The proton at H-22 (δH 3.79), coupled with the proton at H-23 (δH 4.77) in the COSY, indicated the two hemiacetal carbons at C-21 (δC 109.5) and C-23 (δC 110.4). A combined analysis of HSQC and HMBC data showing 21-OCH3 (δH 3.22, 3H, s), 22-OH (δH, 5.33, s) and 23-OCH3 (δH 3.30, 3H, s) attached to C-21 (δC 109.5), C-22 (δC 77.5) and C-23 (δC 110.5) respectively revealed identical substituted furan ring when compared to yunnanol A. The HMBC correlations networks of 20-OH (δH 4.35, s) to C-20 (δC 80.4), C-21 (δC 109.5), C-17 (δC 46.8), and C-22 (δC 77.5) confirmed the furan ring attached at C-17. According to the above information, the planar structure of 1 was elucidated as shown.
The absolute stereochemistry of the C-17 was inferred by biosynthetic path as 1 was the homolog with compound 6 whose absolute configuration was determined by X-ray diffraction study. The relative stereochemistry of the tetrahydrofuran ring of 1 was achieved with the aid of ROESY experiment, in which, the correlations of H-21/OMe-23, and H-21/H-22 suggested these protons and the methoxy were homolateral of the tetrahydrofuran ring. The ROESY cross-peaks of H-23/OH-20 indicated that they are on the other face of the tetrahydrofuran ring. The structure of 1 was elucidated as shown in Fig. 1.
Walsurobustone B (2), a white amorphous powder, showed a molecular ion peak at m/z 641.2938 [M + Na]+ in the HRESIMS (calcd for C33H46O11Na 641.2937), consistent with the molecular formula C33H46O11, which revealed that 2 possessed five more carbon and two more oxygen than compound 1. The difference part was identified as a 2-methylbutyric ester moiety by analysis of 1H and 13C NMR data (Tables 1 and 2). The correlations of H3-5′/H2-3′; H2-3′/ H-2′; H3-4′/H-2′ in 1H–1H COSY spectra confirmed the presence of 2-methylbutanoate moiety. The location of 2-methylbutyric ester moiety at C-11 was proved by COSY correlation of H-11 (δH 5.16, m)/ H-9 (δH 3.06, d, 11.5 Hz), as well as HMBC correlation from H-11 to C-1′ (δC 174.9). The relative configuration of 2 was established by NOESY experiment. The NOESY correlations H-17/H-21, H-17/ H-22, OH-22/H-23, and H-23/OH-20 indicated the former three protons were on the same side of the tetrahydrofuran ring, while the latter three protons were on another side. The strong cross-peaks of H-11/Me-19 and H-11/Me-30 demonstrated that H-11 was β-configuration. Accordingly, the structure of 2 was assigned as depicted in Fig. 1.
Walsurobustone C (3), a white amorphous powder, had the molecular formula of C27H32O7 as established by the HRESIMS at m/z 491.2048 [M + Na]+ (calcd. 491.2045). The 1H and 13C NMR data (Tables 1 and 2) suggested that the compound 3 was structurally related to walsunoid B [16] as the NMR data of both the compounds are almost similar. The main difference between 3 and walsunoid B is the absence of signals for the hydroxyl at C-11(δH 1.84, 2H, m; δC 19.6) in 3, while C-11 (δH 5.05, 1H, d, 6.2 Hz; δC 66.5) in walsunoid B. Particularly, 23-OMe in 3 was assigned to be α-oriented by comparing the relevant NMR data with a reported compound walsunoid D [16] (δH 5.72 (1H, m) and δC 102.4 of CH-23 in 3; δH 5.73 (1H, brs) and δC 102.5 of CH-23 in walsunoid D). Thus compound 3 was deduced as walsurobustone C (Fig. 1).
Walsurobustone D (4) also exhibited a molecular formula ion peak at m/z 509.2141 [M + Na]+ (calcd for C27H34O8Na 509.2151) in its HRESIMS. The 1H and 13C NMR data of 4 is similar to that of isowalsuranolide [4] except for additional NMR signals (δC 56.5 and δH 3.40) due to a methoxy group and the hydrated double bond in the E ring. The location of the methoxy group was established by the HMBC correlation of methoxy methyl protons to the hemiacetal carbon signal at C-21 (δC 109.8). Furthermore, the HMBC correlations from δH 5.29 (s, –OH) to C-20 (δC 77.3), C-21 (δC 109.8), C-17 (δC 46.1) and C-22 (δC 38.6) indicated that the hydroxyl group δH 5.29 attached to the characteristic quaternary carbon C-20 (δC 77.3). The relative configuration of the tetrahydrofuran ring of 4 was established by analysis of the ROESY spectrum. ROESY correlation between H-17 and H-23 indicated that they were co-facial and arbitrarily assigned as β-oriented. The correlations of Me-18 with OH-20, OH-20 with OMe-23 supported the α-orientation of OH-20 and OMe-23. Thus, the structure of 4 has been determined as showed in Fig. 1.
Walsurobustone E (5) was isolated as a white amorphous powder, and the molecular formula C25H32O6 was assigned through its HRESIMS, at m/z 451.2096 [M + Na]+ (calcd for C25H32O6Na, 451.2091). The 1H and 13C NMR spectra showed signals for five methyls (δH 0.88, 1.07, 1.27, 1.48, and 1.57, each 3H), five methylenes, five methines, four high-field quaternary carbons (δC 48.7, 46.8, 40.4 and 41.4), two double bonds (δC 152.5, 141.3, 127.4, and 134.0) and three carbonyl carbons (δC 208.9, 203.8, and 198.0). These data indicated that 5 has the same core structure as cedrelone [17], except the absence of furan ring signal feature in compound 5. Based on the HSQC and HMBC data analysis, a hydroxyl acetone moiety was revealed by a hydroxylmethyl group C-23 (δC 68.5, δH 4.23, and hydroxyl δH 5.31) attached to carbonyl C-22 (δC 208.7) (Fig. 2). The carbonyl C-22 linked to C-17 via C-20 was established by the HMBC correlations from H-17 (δH 2.06) to C-20 (δC 37.7) and C-22 (δC 208.7), and H-16a (δH 1.46) to C-20. Accordingly, the structure of 7 was assigned as depicted in Fig. 1.
Toonapubesic acid B [18] (6) was obtained as a colorless crystal (recrystallized in MeOH/CH3COCH3) and the molecular formula C23H28O6 was assigned through its HRESIMS, at m/z 423.1777 [M + Na]+ (calcd for C23H28O6Na, 423.1783), suggesting 10 degrees of unsaturation. The 13C NMR spectrum (Table 2) of 6 were very close to those for 5, except that the furan ring was oxidized to a carboxylic acid in compound 6. The inferred structure was further established through analysis of its MS and HMBC data. In the HMBC spectrum, H-17 (δH 2.54) and H-16β (δH 2.11) were correlated with the carbon resonance at δC 178.8, which was assigned to the C-20 carboxyl group. According to the above information, the planar structure of 6 was elucidated as shown (Fig. 3A).
The relative configuration of 6 was determined by ROESY spectrum (Fig. 3B). As shown in ROESY data, the correlations of H3-18/H-9, H3-18/H-16α, and H3-18/H-11α indicated that they were co-facial, arbitrarily assigned as α-orientated. The ROESY correlations of H3-30/H3-19, H3-19/H3-29 revealed that H3-30, H3-19, and H3-29 were β-oriented, thus H3-28 was α-orientated. The configuration of five methyls was the same as other reported limonoids [4]. In order to fix the orientation of the epoxide, 6 was recrystallized in methanol: acetone = 1: 1 mixture solvent and been structurally determined by X-ray diffraction analysis (Fig. 4). The X-ray diffraction data were collected by using CuKα radiation and its absolute configuration has been determined as 8R, 9R, 10R, 13S, 14R, 15R, and 17R. Although the structure of toonapubesic acid B (6) was reported in literature [18], while its NMR data was not available. Here we report the X-ray structure of toonapubesic acid B and its NMR data together to facilitate the community.
Hypothetic biosynthesis of seco-E ring derivatives 5 and 6 was proposed based on the common structural characteristics of discovered intermediates (Scheme 1). The furan ring of cedrelone was oxidized to form butenolactone, which was hydrolyzed to give free carboxyl and hydroxyl groups subsequently. After decarboxylation and a few steps oxidation, 5 and 6 formed respectively.
The similar patterns of Cotton Effects in the CD spectra of walsurobustones A-E (1–5) and toonapubesic acid B (6) (Fig. 5) indicated that the former’s chiral centers in rings A, B, C and D have absolute configurations identical to the latter.
The cytotoxicity of 1–6 against the cancer cell lines (HL-60, SMMC-7721, A-549, MCF-7, and SW480) were evaluated by using the MTT method with taxol and cisplatin as the positive control. While compound 6 only showed moderate effects for all cell lines, other five compounds all showed obvious activity, especially to SMMC-7721, A-549 and MCF-7 (Table 3).
3 Experimental section
3.1 General experimental procedure
Optical rotations were measured with a Horiba SEPA-300 polarimeter. UV spectra were detected on a Shimadzu UV-2401 spectrophotometer. IR spectra were determined on a Tenor 27 spectrophotometer with KBr pellets, whereas CD spectra were recorded with an Applied Photophysics Chirascan spectrometer. ESIMS and HRESIMS were measured on a Finnigan MAT 90 instrument and VG Auto Spec-3000 spectrometer, respectively. 1D and 2D NMR spectra were recorded on Bruker AM-400, Bruker DRX-500 and Bruker Avance III 600 spectrometers with TMS as internal standard. Semipreparative HPLC was performed on an Agilent 1100 liquid chromatographer with a Zorbax SB-C18 (9.4 mm 25 cm) column. Column chromatography was performed with silica gel (300–400 mesh, Qingdao Marine Chemical, Inc., Qingdao, People’s Republic of China), and MCI gel (75–150 μM, Mitsubishi Chemical Corporation, Tokyo, Japan). Fractions were monitored by TLC, and spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in EtOH.
3.2 Plant material
The leaves of W. robusta collected in Hainan Province, People’s Republic of China in December 2010. The plant was authenticated by Dr. Guangwan Hu, Kunming Institute of Botany, Chinese Academy of Sciences. A voucher specimen (No. H20101202) was deposited in the State Key Laboratory of Photochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
3.3 Extraction and isolation
The dried and powdered leaves (12 kg) of W. robusta were extracted with MeOH three times under reflux, and the solvent was evaporated in vacuo. The residue was partitioned in H2O and extracted successively with petroleum ether and EtOAc. The EtOAc fraction (200 g) was separated by silica gel column chromatography (CC) eluted with a gradient of petroleum ether/Me2CO (50:1 to 1:1) and CHCl3/MeOH in a gradient (15:1 to 3:1), eight fractions (Fr. A-H) were obtained according to TLC monitor. Fr. C (17 g) was subjected to MCI-gel column (MeOH/H2O, 6:4 to 9:1) to give sixteen sub-fractions (C1–C16). C7 (4.1 g) was then chromatographed on a column of reversed-phase C18 silica gel eluted with MeOH/H2O (5:5 to 9:1) to get eight parts (C7a–C7h). C7h was purified by CC over silica gel and then applied to a Sephadex LH-20 column using a solvent system acetone to provide 1 (5 mg), 3 (5 mg), 4 (11 mg), 5 (15 mg). C8 was further purified by preparative RP-8 HPLC using a solvent system 60% aqueous MeOH to provide compounds 2 (11 mg). Fr. D (60 g) was subjected to MCI-gel column (MeOH/H2O, 4:6 to 9:1) to give twenty fractions (D1–D20), D15 (1 g) was subjected to silica gel column chromatography eluting sequentially with petroleum ether/EtOAc to afford 11 fractions, the second portion was purified by Sephadex LH-20 column to obtain 6 (11 mg).
Walsurobustone A (1), a white amorphous powder; [α] − 30.6275 (c 0.17, CHCl3); UV (CHCl3) λmax (logε) 283 (3.10) nm; 1H NMR (DMSO) and 13C NMR (DMSO) (see Tables 1 and 2). IR νmax cm−1: 3430, 1630 cm−1; HRESIMS at m/z 509.2141 [M + Na]+ (calcd for C27H34O8Na, 509.2151).
Walsurobustone B (2), a white amorphous powder, [α] − 20.25(c 0.16, CHCl3); UV (CHCl3) λmax (logε) 283(3.10) nm; 1H NMR (DMSO) and 13C NMR (DMSO) (see Tables 1 and 2). IR νmax cm−1: 3426, 1682, 1630, 1030, 998 cm−1; HRESIMS at m/z 641.2938 [M + Na]+ in the HRESIMS (calcd for C33H46O11Na, 641.2937).
Walsurobustone C (3), a white amorphous powder; [α] − 21.1905 (c 0.14, CHCl3); UV (CHCl3) λmax (logε) 281 (3.30) nm; 1H NMR (CDCl3) and 13C NMR (CDCl3) (see Tables 1 and 2). IR νmax cm−1: 3406, 2957, 2923, 1764, 1676, 1034 cm−1; HRESIMS at m/z 491.2048 [M + Na]+ (calcd for C27H32O7Na, 491.2045).
Walsurobustone D (4), a white amorphous powder; [α] − 68.6111 (c 0.12, CHCl3); UV (CHCl3) λmax (logε) 283 (3.12) nm; 1H NMR (CDCl3) and 13C NMR (CDCl3) (see Tables 1 and 2). IR νmax cm−1: 3431, 1630 cm−1; HRESIMS at m/z 509.2141 [M + Na]+ (calcd for C27H34O8Na, 509.2151).
Walsurobustone E (5), a white amorphous powder; [α] − 54.5641 (c 0.14, CHCl3); UV (CHCl3) λmax (logε) 281 (3.28) nm; 1H NMR (CDCl3) and 13C NMR (CDCl3) (see Tables 1 and 2). IR νmax cm−1: 3482, 3406, 2924, 2854, 1718, 1680, 1356, 1249, 1031 cm−1; HRESIMS m/z 451.2103 [M + Na] + (calcd for C23H28O6Na, 451.2096).
Toonapubesic acid B (6), a colorless crystal, deposited to CCDC with No.2235264; [α] − 79.4872 (c 0.13, CHCl3); UV(CHCl3) λmax (logε) 280 (3.26) nm; 1H NMR (CDCl3) and 13C NMR (CDCl3) (see Tables 1 and 2). IR νmax cm−1: 3418, 2926, 1713, 1686, 1240, 1031 cm−1; HRESIMS m/z 423.1777 [M + Na]+ (calcd for C23H28O6Na, 423.1783).
References
Luo J, Sun YP, Li QR, Kong LY. Research progress of meliaceous limonoids from 2011 to 2021. Nat Prod Rep. 2022;39:1325–65.
Tan QG, Luo XD. Meliaceous limonoids: chemistry and biological activities. Chem Rev. 2011;111:7437–522.
Peng H, Mabberley DJ. Flora of China. 2008;11:119–20.
Luo XD, Wu SH, Ma YB, Wu DG. Tetranortriterpenoids from Walsura yunnanensis. J Nat Prod. 2000;63:947–51.
Balakrishna K, Rao RB, Patra A, Ali SU. Constituents of Walsura piscidia. Fitoterapia. 1995;66:548.
Chatterjee A, Kundu AB, Chakrabortty T, Chandrasekharan S. Structure of walsurenol, a new pentacyclic triterpene alcohol from Walsura tubulata. Chem Commun 1968;418–9.
Govindachari TR, Kumari GNK, Suresh G. Triterpenoids from Walsura piscidia. Phytochemistry. 1995;39:167–70.
Lakshmi S, Subramanian K, Steiner T, Varghese B. Piscidinol-C, a triterpenoid from Walsura piscidia leaves. J Chem Crystallogr. 1999;29:1141–3.
Purushothaman KK, Duraiswamy K, Connolly J, Rycroft DS. Triterpenoids from Walsura piscidia. Phytochemistry. 1985;24:2349–54.
Yin S, Wang XN, Fan CQ, Liao SG, Yue JM. The first limonoid peroxide in the meliaceae family: Walsuronoid A from Walsura robusta. Org Lett. 2007;9:2353–6.
Zhou ZW, Yin S, Zhang HY, Fu Y, Yang SP, Wang XN, Wu Y, Tang XC, Yue JM. Walsucochins A and B with an unprecedented skeleton isolated from Walsura cochinchinensis. Org Lett. 2008;10:465–8.
Balakrishna K, Sukumar E, Connolly JD. Piscidenone and Piscidinol G, Two new protolimonoids from Walsura piscidia. Nat Prod Sci. 2003;9:192–4.
Luo XD, Wu SH, Ma YB, Wu DG. Chemical constituents from Walsura yunnanensis. Acta Bot Yunnanica. 2001;23:515–20.
Yang W, Kong LM, Li SF, Li Y, Zhang Y, He HP, Hao XJ. Five new mexicanolide type limonoids from Heynea trijuga. Nat Prod Bioprospect. 2012;2:145–9.
Cao MM, Zhang Y, Li XH, Peng ZG, Jiang JD, Gu YC, Di YT, Li XN, Chen DZ, **a CF, He HP, Li SL, Hao XJ. Cyclohexane-fused octahydroquinolizine alkaloids from Myrioneuron faberi with activity against hepatitis C virus. J Org Chem. 2014;79:7945–50.
Wang GC, Yu JH, Shen Y, Leng Y, Zhang H, Yue JM. Limonoids and triterpenoids as 11beta-HSD1 inhibitors from Walsura robusta. J Nat Prod. 2016;79:899–906.
Mohankumar R, Ilango K, Pridiuldi V, Santhanakrishnan, Radhakrishnan V, Narasimhan S. Ethylenediamine: an effective reagent for deacetylation of natural products. J Asian Nat Prod Res. 2010;12:851–8.
Wang JR, Liu HL, Kurtán T, Mándi A, Antus S, Li J, Zhang HY, Guo YW. Protolimonoids and norlimonoids from the stem bark of Toona ciliata var. pubescens. Org Biomol Chem. 2011;9:7685–96.
Acknowledgements
This research was supported financially by grants from the National Natural Science Foundation of China (U1812403 to X.-J. Hao and 22177050 to M. Cao), Project of Yunnan Characteristic Plant Screening and R&D Service CXO Platform (2022YKZY001), Foundation of Central Asian Drug Discovery and Development Center of Chinese Academy of Sciences (CAM202103, China), and Youth Innovation Promotion Association CAS to M. Cao (2022-2026).
Author information
Authors and Affiliations
Contributions
Li Hou carried out the isolation and data curation at leading degree. Cui-Xuan Mei conducted the writing of original draft. Chun-Mao Yuan contributed to investigation, and validation at supporting degree. Gui-Hua Tang contributed to investigation, and validation at supporting degree. Duo-Zhi Chen contributed to data curation and analysis at supporting degree. Qing Zhao contributed to project administration at supporting degree. Hong-** He contributed to project administration at leading degree. Mingming Cao contributed to guiding of the writing and data proof reading. **ao-Jiang Hao contributed to funding acquisition and project administration at leading degree. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare there is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hou, L., Mei, CX., Yuan, CM. et al. Five new limonoids isolated from Walsura robusta. Nat. Prod. Bioprospect. 13, 7 (2023). https://doi.org/10.1007/s13659-023-00371-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13659-023-00371-6