Abstract
The efficiency of classical mineral NPK fertilizers is usually low because a major part of these fertilizers does not reach plant roots and ends up polluting groundwaters with nitrates and phosphates. Recently, a novel polymer-coated urea made from recycled plastics was proposed to enhance N availability in cereal production. To evaluate the efficiency of this polymer for rice production, we set up field plots, microplots, and pot experiments with 15N tracing. We compared rice yield, N uptake, and N loss between conventional three split applications of urea and a single basal application of four derivatives from the polymer-coated urea. The four derivatives included a blend with 70 % of N from 6 % (w/w) coated urea and 30 % from urea and three coated urea fertilizers with 6, 8, and 12 % coating at an identical N application rate during two rice-growing seasons. Results show that 6 % coated urea improved 15N recovery, reduced 15N loss, and increased grain yield slightly due to an initial 15N burst occurring at high field temperatures after basal fertilization; 8 or 12 % coated urea better met plant N demand from transplanting to heading, greatly enhanced 15N recovery, and decreased 15N loss and NH3 volatilization. Nevertheless, unlike a significant increase of yield for 12 % coated urea, 8 % coated urea did not increase yield due to 15N release and excessive 15N uptake by plants at ripening. Overall, our findings show that a single basal polymer-coated urea application improves N use efficiency and reduces N loss in rice agroecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Synthetic N fertilizers have played an irreplaceable role in crop yield increase and food security for rapid population growth since the Haber-Bosch process was invented in the early twentieth century (Smil 1999). China is the largest consumer of synthetic N fertilizer in the world. Since 2010, synthetic N fertilizer use in Chinese agriculture has exceeded 3.19 million tons N/year (National Bureau of Statistics of China 2011), accounting for more than one third of the total world consumption (FAO 2008). However, the current efficiency of N fertilizer use is very low, and losses are large. It is estimated that average N use efficiency by cereal crops is only 35 %, and losses of N fertilizer can be as high as 52 %. In terms of total N loss, the reactive N accounts for 19 % of the N applied with 11 % lost via NH3 volatilization, 5 % via runoff, 2 % via leaching, and 1 % via N2O emission (Zhu and Chen 2002). These considerable amounts of reactive N enter the environment and pollute both the atmosphere and water systems (Galloway et al. 2008). Consequently, strategies for improving the efficiency of fertilizer N use, and optimizing the relationship between agricultural N management and environmental quality, have attracted the attention of agricultural scientists.
Other than through innovations in breeding and cultivation and fertilization techniques, one possible way to improve N use efficiency while reducing the environmental impacts is by using slow- or controlled-released fertilizers instead of conventional, readily soluble N fertilizers (Zhu et al. 1997). Slow- and controlled-released fertilizers have the capacity to delay the availability of N for plant uptake or to extend its availability to the plant longer than “readily available N fertilizers” such as urea, ammonium bicarbonate, or ammonium sulfate (Trenkel 1997; Shaviv 2001). However, with slow-released fertilizers such as urea formaldehyde and isobutylidene diurea, the N release patterns, rates, and duration are strongly dependent on variable soil properties, in particular biological activity and external conditions such as moisture content, wetting and drying, and temperature (Aarnio and Martikainen 1995; Trenkel 1997); thus, their effects on agriculture cannot be consistent and predictive (Shaviv 2001). In contrast, the characteristics of N release from controlled-released fertilizers such as sulfur- or resin-coated urea are quite predictable, because these fertilizers are reported to be relatively unaffected by soil properties, and the N release longevity can be controlled by using organic/inorganic polymers that are thermoplastics or resins or sulfur as a physical diffusion barrier, and altering the ratio of components in the coatings (Shaviv 2001). Numerous studies have addressed the advantages of controlled-released fertilizers in reducing N losses to the environment, e.g. leaching, gaseous emissions and increasing N use efficiency (Trenkel 1997; Chien et al. 2009). Moreover, the rate of N application or the number of applications during the growing season can often be reduced, which has the added advantage of saving in labor costs. Nevertheless, the cost of manufacturing sulfur- or resin-coated urea is at least two to four times that of conventional mineral N fertilizers (Watson 2013). Thus, the cost-benefit ratio at present prevents their wide use in general cereal production, in contrast to non-agricultural markets or high value crops. Currently, the use of controlled-released fertilizers in Chinese agriculture is limited to only 0.4 million tons per year, and it accounts for less than 6 % of total national fertilizer consumption (Watson 2013).
Recently, a new type of polymer-coated urea that uses thermoplastic resin was developed in China (Zhang et al. 2006). The main coating resin of this fertilizer is made from recycled plastic films which were initially used for greenhouses on local vegetable farms. This material can reduce the coating material cost by nearly 70 % compared with using new polymer coating material (Yang et al. 2012a). Because of the relatively low price and ease of application (Yang et al. 2011), large-scale production of derivatives from this type of polymer-coated urea have been achieved by the Shandong Kingenta Ecological Engineering Co. Ltd (Linshu, China), using a fluidized bed boating process and is being extended to the broader Chinese fertilizer market. There is also growing evidence that a single application of this polymer-coated urea fertilizer with varying N release longevities (different formulations can be made by varying the coating contents) could increase apparent N use efficiency, which is calculated from the difference in N uptake between the plot receiving N and the no-N addition control (Zhu et al. 1997) by 23–104 % at the same N rate compared with the normal urea split applications in the three major grain crops, rice (Yang et al. 2012b, 2013), wheat (Yang et al. 2011), and corn (Ning et al. 2012). These improved N use efficiencies resulting from use of the polymer-coated urea also produced yield increases of 3.23–26.5 %. However, it is worth noting that in these studies, the yield response to the polymer-coated urea application may not always be positively correlated with enhanced N use efficiency. For example, Yang et al. (2012b) observed no significant increase in rice yield accompanying a 25.7 % enhancement in N use efficiency compared with the urea control at the same normal N rate of 300 kg N ha−1 in the first year, although yield greatly increased by 18.4 % in the second year with a somewhat lower increase of 22.9 % in N use efficiency. This result hints at the necessity for a detailed investigation into the relationship between N uptake by crop plants and N release from polymer-coated urea over the entire crop-growing season, which could provide an explanation for the crop yield differential under improved N use efficiency, as well as important information for a certain polymer-coated urea’s capacity to match nutrient supply with plant demand. Although Yang et al. (2012b) observed good consistency between N accumulation in the rice plant and cumulative N release of polymer-coated urea under field conditions, it should be noted that there is still a lack of direct evidence addressing the synchronization of N release from polymer-coated urea and plant N demand because there is no differentiation between plant N obtained from fertilizer and from the soil. In this case, the 15N tracing technique is very useful for examining the patterns of N release from polymer-coated urea and plant N uptake, with the additional benefits of allowing for an accurate estimate of N recovery, residual N, and total N loss from polymer-coated urea (Kamekawa et al. 1990; Inubushi et al. 2002).
The Taihu Lake region is one of four major rice-based agroecosystems located in the central Yangtze River Delta of subtropical China, which is the most intensive agricultural and the most economically developed area. This region is currently characterized by a high summer rice yield of c. 7500 kg ha−1, high application rates, and frequencies of readily soluble synthetic N fertilizer at c. 240–300 kg N ha−1 in total with three splits per rice season. Unfortunately, the efficiency of chemcial N fertilizer use in rice system here is generally very low, with less than 30 % of the applied chemical fertilizer actually being used by the growing crop and more than 50 % N actually being lost to environment (Zhao et al. 2012). At present, there is little information describing the effects of a single basal application of the abovementioned type of polymer-coated urea instead of the conventional split applications of urea to maintain rice yield and reduce N loss in this rice ecosystem. In view of the high-temperature and high-humidity conditions for rice growth in this region due to the subtropical climate, we considered that the polymer-coated urea may have a potential for future use here. However, the formulations of the polymer-coated urea need to be optimized, and are largely different from that which has already been proven efficient in the rice ecosystems of northeast and central China (Yang et al. 2012b, 2013; Lin and Li 2007).
Thus, the objectives of our research were (1) to evaluate the yield response, NH3 volatilization, and NH4 + concentrations in flood water following applications of four derivatives from the polymer-coated urea with varying coating contents using field plots; (2) to determine the effects of these polymer-coated urea fertilizers on N use efficiency and total N loss using field 15N microplots; and (3) to explore whether it is possible to synchronize the N release pattern from polymer-coated urea with plant N uptake patterns by pot experiment using 15N-labeled fertilizers and destructive sampling. The information obtained from this study could provide more insight into the agronomic and environmental effects of this novel polymer-coated urea in rice paddies, so that the optimal formulation of the polymer-coated urea can be adopted for rice production in the Taihu Lake region of China.
2 Materials and methods
2.1 Paddy soil and N sources
The study was performed during the 2008 and 2009 rice-growing seasons at the Yixing Base for Agri-Environment Research, Changshu National Agro–Ecosystem Observation and Research Station, Chinese Academy of Sciences. This station is located on the northwest side of Taihu Lake, within 1 km of the shore (Fig. 1a) and has a subtropical monsoon climate with an average temperature of 15.7 °C and an annual rainfall of 1177 mm. The paddy soil here is typical fluvaquent, classified by USDA Soil Taxonomy and originated from a Lacustrine deposit as parent material. The top 15-cm soil layer in the fields consists of 8.3 % sand, 81.5 % silt, and 10.2 % clay by volume and contains 15.4 g kg−1 organic C and 1.79 g kg−1N. The cation exchange capacity and the pH (H2O) of the soil (top 0–15-cm layer) were 11.8 cmol kg−1 and 5.6, respectively.
Location of experimental field site (a), views of field plots (b, c), 15N microplots (b, d), and 15N pot experiments (e). We performed a sequence of experiments and used the 15N tracing method to compare rice yield, plant N uptake, and N loss between conventional three split applications of urea and a single basal application of four derivatives from polymer-coated urea at an identical NPK application rates over two rice-growing seasons
A group of field plots, 15N microplots, and 15N pot experiments were conducted in this study (Table 1; Fig. 1). For the field plot experiment, a blend product with polymer-coated urea and non-coated urea and three polymer-coated urea products with varying coating contents by weight were obtained from the Shandong Kingenta Ecological Engineering Co. Ltd (Linshu, China) (Table 1). The N contents of 6, 8, and 12 % coated urea fertilizers were 43.2, 42.3, and 40.5 %, respectively. These thermoplastic polymer-coated urea types are characterized by a semipermeable membrane that enables water to enter the granule and to dissolve the N inside. The theoretical release longevity of 6, 8, and 12 % coated urea fertilizers were 90, 120, and 180 days in water at 25 °C, respectively (Yang et al. 2013). The conventional urea product was 46.3 % N, with particle sizes ranging from 1.2 to 2.0 mm (Wuxi Ligu Chemical Co. Ltd.; Yixing, China). For the microplot and outdoor pot experiments, 15N-labeled urea was used in powder form with 46.0 % N and 10.22 % 15N abundance, and was obtained from the Shanghai Engineering Research Center of Stable Isotope. 15N-labeled urea prills with particle size 1.0–2.0 mm were prepared at laboratory scale by adding the urea powder into a melt granulating steel dropper at 120–125 °C assembled with liquid coolant. The 15N-labeled coated urea fertilizers were prepared using the same coating materials and proportioning and by a similar coating process that was adopted for production of the corresponding commercial products from Shandong Kingenta Ecological Engineering Co. Ltd. Briefly, the 15N-labeled urea prills were loaded into a rotating (30–40 rpm) steel cylinder heated to 70–75 °C. The recycled thermoplastic resin coating materials were then evenly deposited onto the surfaces of the rotating urea prills. The curing reaction of the mixed coating material was finished in 8 min. The weight of the thermoplastic resin material coating accounted for approximately 1 % of that of the urea fertilizer in each coating process. The 15N-labeled 6, 8, and 12 % polymer-coated urea were produced by repeating the coating process 6, 8, and 12 times, respectively. The N contents of the three 15N-labeled coated urea fertilizers were 43.0, 42.0, and 40.1 %, respectively, which were very similar to those from the commercial products mentioned above.
The rice cultivar used in this study was Zhendao-10, a popular japonica rice cultivar with growing period of ∼120 days, planted in paddy soils with moderate soil fertility in the Taihu Lake region.
2.2 Field plot trial
The total N input per rice season was the same for the 2-year plot experiment (Fig.1b) with 240 kg N ha−1. The treatments in the 2-year field trial were shown in Table 1. For all the coated urea treatments in the two rice seasons, the N fertilizers were basally applied one time during transplanting of the rice seedlings by incorporation into the top 15-cm layer of the paddy soil without any topdressing. For the urea treatments, 30 % of N was incorporated into the top 15 cm of the paddy soil at the seedling as basal fertilization, the remaining 70 % was surface applied and split at a 4:3 ratio for topdressing at tillering and ear differentiation stages. P and K fertilizers were consistently applied basally at a rate of 60 kg P2O5 ha−1 and 45 kg K2O ha−1, respectively.
Rice seedlings are usually transplanted in late June at a density of 25 hills m−2 with four plants in each hill and manually harvested in late October or early December. Flood water was mostly maintained at a depth of 3–5 cm in the field except for an approximate 1-week midseason aeration at elongation and a final drainage event at maturity.
NH3 volatilization was measured using a continuous air flow enclosure method (Kissel et al. 1977). A chamber for measuring NH3 volatilization were installed in each plot (Fig. 1c). The volume of the volatilization chamber with 200 mm in diameter could be adjusted by changing the depth to which the chamber is inserted into the soil or water. The rate of air flow was set to 15–20 times that of the chamber volume per minute using a pump. For the urea treatments, NH3 volatilization was measured twice each day (08:00 to 10:00 and 14:00 to 16:00) after each urea application for more than 1 week until NH3 was no longer detected (Zhao et al. 2012). For the coated urea treatments, NH3 volatilization was observed for two time periods: the first one was lasting for approximately 1 month after the single basal fertilization, and the second one was lasting for 1 week coninciding with the measurement for the second topdressing in the urea treatments. The samplings was the same as for the urea-treated plots, as twice each day (08:00 to 10:00 and 14:00 to 16:00). When each sampling was taken, the soil and air temperature were measured simultaneously. The NH3 captured from the air was continuously pumped through an adsorbent solution containing 2 % H3BO3 mixed with an indicator composed of methyl red, bromocresol green, and ethanol for 2 h for each measurement. This adsorbent solution was titrated with a predetermined acid solution to determine the amount of trapped NH3. The total NH3 volatilization flux was calculated as the sum of daily volatilization rates over the period.
The flooded water in each plot was collected using a 50-ml syringe coninciding with the measurement of NH3 volatilization. Flooded water samples were stored in 100 ml plastic bottles and immediately frozen without filtering at −20 °C until analysis.
Rice plants from each field plot were manually harvested. The fresh weight of the grain from the entire plot was weighed after machine threshing. Harvested grain samples were dried at 70 °C for 48 h for dertimination of moisture content. Per-unit-area yield of grain was then computed by deducting the moisture contents from the fresh weights.
2.3 Field 15N microplot trial
The treatments for the 15N microplot trial (Fig. 1d) were the same as for the field plot trial (Table 1). However, the aforementioned 15N-labeled urea and coated urea fertilizers were used to replace unlabeled commercial N sources with an identical dose of NPK fertilizers; 240 kg N ha−1, 60 kg P2O5 ha−1, and 45 kg K2O ha−1 for each microplot. The microplots were cultivated under the same fertilization and water management practices as for the corresponding field plot experiments.
Rice plants from each microplot were manually harvested at the soil surface with no stubble left standing above ground. The fresh weight of the grain was weighed after machine threshing. Harvested plant samples were dried for analysis of the grain/straw dry weight, N, and 15N accumulation. At the end of each rice harvest, the top 0–15-cm soil layer was sampled in each microplot by taking five cores with 2 cm in diameter, which were then air-dried and sieved through a 0.25-mm mesh after picking out residual coated urea granules for analysis of the soil N content and 15N aboundance. 15N recovery was calculated as total 15N accumulation in the aboveground parts of the rice plant divided by the total 15N input into each microplot. The residual 15N amount was computed based on the measured soil density of 1.13 g cm−3. 15N residual rate was calculated by dividing the total residual 15N amount in 0–15 cm soil by the total 15N input. The rate of total 15N loss was estimated from the difference between 100 % and the measured 15N recovery and residual rate (Zhu et al. 1997).
2.4 15N pot experiment
The pots (Fig. 1e) were loaded with 8 kg of the same 2-mm-sieved paddy soil by dry-weight basis, which was used for the field plot and microplot experiments. For the coated urea-treated pots (Table 1), the 15N-labeled coated urea samples were placed in a 25 × 25-cm polypropylene mesh bag with 0.8 mm2 mesh size and laid flat on the soil surface (Shoji 1999; Wilson et al. 2009). Each bag contained 1.68 g N, being equivalent to 240 kg N ha−1 that was used for the field plot and microplot experiments. Afterwards, the bag was buried by addition of another 12 kg paddy soil mixed with 0.42 g P2O5 and 0.32 g K2O of PK fertilizers, being equivalent to 60 kg P2O5 ha−1 and 45 kg K2O ha−1 that was used for the field plot and microplot experiments. The soil was packed at a density of 1.13 g cm−3 under field condition and at a depth of 25 cm. For 15N-labeled urea-treated pots, 12 kg paddy soil mixed with 0.50 g basal N (30 % of total N input) and PK fertilizers was directly packed into the pot at the same soil density. The remaining 1.18 g N (70 % of total N input ) was surface-applied and split at a 4:3 ratio for topdressing during the tillering and ear differentiation stages, respectively.
Rice seedlings were transplanted to the pots in late June at the same density as in the field plots and were harvested in late October, 2009. During the rice-growing season, the soil remained flooded to a depth of 5 cm, except for a midseason aeration at rice elongation and a final drainage at rice maturity until harvest. Three pots from each treatment were taken on day 16 after transplanting, elongation on days 31, booting on days 41, heading on days 82, maturity on days 111, and harvest stages on days 120 of rice growth, respectively. Rice plants were also harvested with no stubble standing above ground. Harvested plant samples were dried for analysis of the grain/straw dry weight, N, and 15N contents. 15N uptake rate (mg N pot−1 day−1) was calculated by the net increases in 15N in the aboveground parts during the adjacent critical rice growth stages divided by the day-time intervals.
The buried bag in the pot soil was removed at each sampling, rinsed with distilled water to remove the attached soil from the granules and sampled to determine the residual N and 15N (Shoji 1999; Wilson et al. 2009). The cumulative amount of N released from each coated urea during each rice growth period was estimated as the difference between the total 15N input and the respective residual 15N in the coated urea divided by the total 15N input. The 15N release rate (mg N pot−1 day−1) was calculated by the net increase in 15N release during the adjacent critical rice growth stages divided by the day-time intervals.
2.5 Physicochemical and statistical analyses
Soil basic properties, soil and plant N, and 15N contents were determined following standard soil agro-chemical analytical procedures (Lu 2000). Soil pH was measured in a 1:2.5 (w/v) soil-to-water mixture using a pH meter. Soil organic C was determined by the wet-digestion method using potassium dichromate with external heat and back titration to measure the unreacted dichromate which serves as the proxy for the C content. Soil CEC was measured using the ammonium acetate method. Briefly, it was measured at soil pH 7 after displacement using the 1 M NH4-acetate method followed by titrimetric calculation via the distillation of ammonium. Particle size distribution of the soil was quantified with a laser particle characterization analyzer (Beckman Coulter, Brea, CA, USA).
N contents of soil, grain, and aboveground straw were measured using the Kjeldahl method. 15N abundance in soil and plants was determined using an isotope mass spectrometer (with analytic error ±0.02 %; MAT-251, USA). NH4 + concentration in samples of flooded water was analyzed with a continuous flow analyzer (with analytic error ±3.9 % and low detection limit of 0.2 mg N L−1; Skalar, The Netherlands).
All figures were drawn with Originpro 8.5 software (OriginLab, USA). Statistical analysis of the data was conducted with SAS 8.1 software (SAS Institute Inc., USA). One-way analysis of variance (ANOVA) was performed to assess the significance of differences in parameters between different treatments in a certain rice season. Lowercase letters in the table indicate statistically significant differences after the least significant difference (LSD) test at p < 0.05.
3 Results and discussion
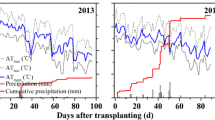
For the blend treatment with 6 % coated urea and urea, 30 % urea-N was initially suggested to partially substitute for coated urea-N, based on the notion that it is necessary to blend readily soluble N fertilizer with a single basal application of polymer-coated urea. This is because the normal lag period of N release in polymer-coated urea would restrict the early N requirement for the rapid establishment of transplanted rice seedlings (Shoji 1999). But contrary to the initial assumption, no difference (p < 0.05) was found in rice yield or 15N recovery and loss between the blend treatment and the urea treatment (Table 2). This result disagrees with the previous results of Lin and Li (2007) in paddy soils from northeast and central China using the same N fertilizer sources. The reasons might be attributed to greater N loss via NH3 volatilization driven by superfluous NH4 + accumulation in flood water beyond the requirement of the transplanted rice seedings in the early stages of growth after basal fertilization. For flooded rice, NH3 volatilization during the early growth stages is an important gaseous loss limiting fertilizer N use efficiency due to the heavy NH4 +-based fertilization under flood conditions, strong sunlight, and high temperatures in the rice field (Zhu and Chen 2002; Cai et al. 1988). NH3 volatilization is directly proportional to NH4 + concentration in flood water (Zhu et al. 1997). The blend treatment had much higher NH4 + concentrations in the flood water relative to the urea treatment during the week following rice transplanting (Fig. 2c) despite the fact that both treatments received the same dose of readily soluble urea (72 kg N ha−1; 30 % of total N input). Apparently, an initial N release occurred in the coated urea fraction of the blend treatment. The relatively high concentrations of NH4 + in flood water under the 6 % coated urea treatment for the same time period (Fig. 2c) also supports this explanation. As a consequence of significant NH4 + accumulation in flood water, NH3 volatilization during the 20 days following basal fertilization was increased by 1.5 times (p < 0.05) in the blend treatment compared with the urea control (Table 2). These results suggest that blending readily soluble urea with basal polymer-coated urea application was unnecessary in the study region, and it was not only useless for improving N use efficiency and rice yield, but also would increase N loss to the environment during the early stages of rice growth.
Temperatures of air and soil (a, b), concentration of NH4 + in flood water (c, d) over an approximate 2-month period after rice transplanting under conventional three split applications of urea and a single basal application of polymer-coated urea fertilizers in the 2008 and 2009 rice-growing seasons. Concentration values of NH4 + are means of triplicates ± standard deviation. Arrows denote the three split applications of urea. Blended urea and 6 % (w/w) coated urea increased NH4 + concentrations in the flood water during the week after rice transplanting, compared with the urea treatment. The three coated urea fertilizers showed a decreasing trend in flood water NH4 + concentrations in the 20 days after fertilization with increasing coating contents from 6 to 12 %
The 6 % coated urea treatment increased rice yield by 4.73 % (p < 0.05) accompanied by a 44.5 % increase in plant 15N recovery (p < 0.05) compared with the urea treatment (Table 2). This result indicated the potential validity of using polymer-coated urea to improve yield and fertilizer use efficiency in lowland rice (Shoji 1999; Shaviv 2001). The results are comparable with those of Yang et al. (2012b, 2013), who used similar N sources in a paddy field in northern China and found that at the same N rate of 100–300 kg N ha−1, a single basal polymer-coated urea application produced 22.9–78.9 % increases in total apparent N uptake efficiency and 3.23–26.6 % increases in rice grain yields compared with the conventional three-split urea applications. A key reason for the positive effect on rice yield and fertilizer use efficiency from 6 % coated urea in the present study is a 31.2 % reduction in total 15N loss (p < 0.05) during the rice season resulting from the significant 51.0 % decrease in NH3 volatilization (p < 0.05) compared with the urea control (Table 2). In particular, the two surface topdressings of N on days 21 and 49 in the urea treatment resulted in high peak levels of NH4 + in flood water and had the potential to increase NH3 volatilization in the middle stage of rice growth from tillering to booting; by contrast, the flood water in the 6 % coated urea treatment could maintain low NH4 + concentrations (<2 mg N L−1) during the time period when topdressings were applied, which explains the decreased NH3 volatilization and 15N loss in the paddy soil receiving 6 % coated urea as compared with urea (Table 2; Fig. 2c).
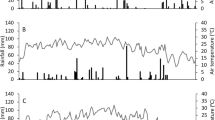
Nevertheless, it is worthy to note that 6 % coated urea still had an unexpected initial quick N release (Fig. 2c)—a so called burst—under actual paddy field conditions (Shaviv 2001), which could result in a certain amount of N loss in the early rice growth stage (Table 2), and inadequate N supply in the middle growth stage, thus lowering to some extent the efficiency of 6 % coated urea in improving N use efficiency and rice yield. The mesh bag method in the pot experiment together with the 15N tracing technique clearly revealed that 6 % coated urea released nearly 86 % of its N in 41 days, with nearly 70 % of the N released (Fig. 3a) and 2.75 mg N day−1 of the highest 15N release rate occurring in no more than 20 days after transplanting (Fig. 3b). As a consequence of the initial high N release rate, 27.6 kg N ha−1 of fertilizer N remained to be lost via NH3 volatilization due to the relatively high concentrations of NH4 + in flood water during the first 20 days after application (Table 2; Fig. 2c). The rate of 15N uptake by rice plants in the 6 % coated urea treatment declined noticeably during the middle stage of rice growth from tillering to booting as compared with the urea treatment, despite the fact that it was greater from rice transplanting to tillering (Fig. 3c). These findings contradicted the notion that polymer-coated urea mostly follows a sigmoidal N release curve with a very slow release stage in water under laboratory conditions at a temperature of 25 °C (Shoji 1999). This discrepancy might result from the large differences in climatic conditions after polymer-coated urea application. For a polymer-coated urea with a certain coating material content, N release is generally dependent on the soil temperature. Increases in temperature would usually increase the rate of N release significantly by increasing the water permeability of the capsule (Kochba et al. 1990; Shaviv 2001). Yang et al. (2012b, 2013) reported an initial 40-day very slow release stage in polymer-coated urea with same coating materials and processes as used in this study after a single basal application in a paddy field in north China. However, the maximum air temperature after basal polymer-coated urea application in the Yang et al. studies was below 25 °C, which is far lower than the temperatures of the air and soil in our study (Fig. 2a, b) that averaged 31.9–32.6 and 31.6–33.7 °C over 1 month in 2008 and 2009 after basal fertilization, respectively. These results in fact show that 6 % polymer-coated urea, which is usually recommended for rice paddies in north China, is not the optimum polymer-coated urea type for improving N use efficiency and rice yield and for reducing environmental risk of N loss in the subtropical Taihu Lake region of southern China, due to its fast initial release rate caused by the relatively high temperatures that persist throughout the summer rice-growing season.
Accumulated 15N release (a), 15N release rate (b), and 15N uptake (c, d) by the aboveground plant parts under a single basal application of polymer-coated urea fertilizers compared with conventional three split applications of urea. Data are means of triplicates ± standard deviation; 6 % polymer-coated urea had a large initial N release and a greater 15N uptake rate from rice transplanting to tillering, but thereafter it became lower than the urea control. Both 8 % polymer-coated urea and 12 % polymer-coated urea reduced initial N release, delayed the occurrence of N release peaks, and formed similar curve shapes for 15N uptake rate compared with the urea control. However, a slight increase in 15N release and greater 15N uptake rates were found in 8 % polymer-coated urea from maturity to harvest
Considering that the increased thickness of the thermoplastic polymer coating has a prolonged effect on the N release longevity of polymer-coated urea (Shaviv 2001), we further examined the effects of single basal 8 or 12 % polymer-coated urea application with increases of 2 or 6 % in the coating contents in the second rice season (2009). As expected, both two polymer-coated urea fertilizers had a reduced initial N release rate compared with 6 % polymer-coated urea (Fig. 3a, b). The respective cumulative 15N release for 8 % polymer-coated urea and 12 % polymer-coated urea was reduced to 21.5 and 15.7 % in days 0–16 from transplanting to tillering; it increased to 39.1 and 15.3 % in days 16–31 from tillering to elongation and 15.9 and 45.6 % in days 31–82 from elongation to heading, respectively (Fig. 3a). The occurrence of the highest peak values for the rates of release of 15N from 8 % polymer-coated urea (2.71 mg N day−1) and 12 % polymer-coated urea (1.75 mg N day−1) were also delayed until 31 and 41 days after rice transplanting, respectively (Fig. 3b). These effective controls to 15N release from 8 and 12 % polymer-coated urea made the curve shapes for the rates of 15N uptake by the rice plants under these two polymer-coated urea treatments similar to that of the urea control (Fig. 3d). This is obviously different from the 6 % polymer-coated urea treatment (Fig. 3c). The greater 15N uptake rates were also observed in the 8 % polymer-coated urea treatment from transplanting to elongation and in the 12 % polymer-coated urea from tillering to elongation than during the corresponding periods in the urea control (Fig. 3d). This could explain the even greater increases in plant 15N recoveries (p < 0.05) in the 8 % polymer-coated urea (86.8 %) and 12 % polymer-coated urea (47.5 %) treatments than in the urea control as compared with the 6 % polymer-coated urea treatment (44.5 %) from the microplot experiment (Table 2). Correspondingly, the 15N loss from 8 % polymer-coated urea and 12 % polymer-coated urea decreased by 42.0 and 22.1 %, respectively (p < 0.05), relative to the urea control. The greater reduction in NH3 volatilization (p < 0.05) in 12 % polymer-coated urea (84.7 %) than in 8 % polymer-coated urea (32.3 %) compared with the urea control (Table 2), closely correlated with NH4 + concentrations in flooded water, which was far lower in the 12 % polymer-coated urea treatment (Fig. 2d) due to the relatively slower release rate (Fig. 3a, b).
Interestingly, the 8 % polymer-coated urea treatment produced a rice yield equivalent to the urea control (p > 0.05) even though it had the highest increase in 15N recovery (p < 0.05) compared with the others; in contrast, the 12 % polymer-coated urea treatment produced the greatest increase in rice yield (8.53 %; p < 0.05) despite the relatively lower enhancement in 15N recovery (p < 0.05) (Table 2). This seemingly contradictory result, in fact, shows that an increase in rice yield not only correlated with improved N use efficiency by the rice plants but also largely correlated with the better synchronization of N release patterns from polymer-coated urea with N uptake by rice plants during the three critical growth stages. In farmer’s fields in the Taihu Lake region, a basal fertilization and two topdressings are necessary to provide enough N to meet the demands of the rice plants at transplanting, from tillering to elongation, and from booting to heading, respectively. In the ripening stage that follows, rice plants absorb little N, in most cases, to complete the translocation of N from leaves or stems to grains (Wada et al. 1986). The reduced yield response from the 8 % polymer-coated urea treatment could be explained by the fact that 8 % polymer-coated urea still showed a slight increase in 15N release (6 % of the input N was released with rates increasing from 0.12 to 0.41 mg N day−1; Fig. 3a, b) in days 82–111. Consistently, greater 15N uptake rates of 0.52–0.66 mg N day−1 in the 8 % polymer-coated urea treatment than in the 12 % polymer-coated urea treatment were observed from maturity to harvest (Fig. 3d), indicating the high level of N absorption by the rice plants at the ripening stage. Under such conditions, no yield advantage resulted from improved N use efficiency by 8 % polymer-coated urea, mainly due to the delay in rice leaves turning yellow and decreased lodging resistance of the rice plants (Zhang et al. 2013). The obviously lower harvest indexes for grain and grain N from the 8 % polymer-coated urea treatment supports this notion (Table 2).
To our knowledge, this study was the first attempt to use field plots, microplots, and pot experiments together with the 15N tracing method to systematicly examine the effects of substituting a single polymer-coated urea application for split urea applications on grain yield, plant N uptake and N loss in intensive rice ecosystem of southeast China (Table 1; Fig. 1). It should be noted that the rice yield data from one crop season in the present study was insufficient for an accurate confirmation about the increasing effect of polymer-coated urea on rice production of the Taihu lake region, mainly due to inconsistencies in the yield responses to different polymer-coated urea types and lack of yearly repeated results. However, the reliabilities of improved N use efficiency and decreased fertilizer N loss, in particular, reduced NH3 volatilization by polymer-coated urea could be warranted, because 6, 8, and 12 % polymer-coated urea types consistently enhanced 15N recovery by rice plants and reduced total 15N loss and NH4 + concentration in flood water (Table 2). In additon, the data of 15N uptake rate and 15N release rate obtained by destructive samplings at six critical growth stages of rice in the pot experiment (Fig. 3) provided strong evidence of variations in the relationship between rice yield and improved N use efficiency by the rice plants as affected by the synchronization between N release and N demand.
Despite the great potential of coated N fertilizer in enhancing N availability and reducing N loss in cereal production, the environmental impact of coating substances spread into the soil should be considered, particularly with long-term repeated high application rates (Shaviv 2001). Using two silt loam soils and lysimeters, Cheng et al. (2011) examined the impacts of one-time incorporation of coating resin from the same polymer-coated urea as in this study at extremely high rates on soil properties and crop growth. They found that 3600 kg ha−1 polymer coating had no significant effect either on soil characteristics and plant nutrients or on grain yields and qualities over a wheat/corn rotation. In fact, this great amount of incorporated polymer coating is relatively very small compared with the huge amount of soil and only accounted for 0.2 % of the total weight of 0--15 cm soil cultivated layer, which is estimated to 1,695,000 kg ha−1 based on a measured soil density of 1.13 g cm−3. Moreover, according to the normal seasonal N application rate—240 kg N ha−1 for rice and the N content in the 12 % polymer-coated urea type—40.5 % in the current study, the soil accumulation of 3600 kg ha−1 polymer coating is equivalent to 51-year repeated applications of 12 % polymer-coated urea regardless of possible photodegradation and biodegradation of the polymer resin. Therefore, we consider that the coating substance is unlikely to have obvisouly negative influence on soil fertility and crop growth in paddy soil of the study region when 240 kg N ha−1 of the current polymer-coated urea per crop season is applied. Nevertheless, the fate of the polymer coating for long-term polymer-coated urea use still need to be addressed. Moreover, development of polymer-coated urea with degradable coatings is of significance to reduce the rate of accumulation of undesired polymers in soil (Shaviv 2001).
4 Conclusions
In the plot and 15N microplot experiments, we observed that 6, 8, or 12 % polymer-coated urea types induced 44.5–86.8 % increases in 15N recoveries by rice plants, 22.1–42.0 % decreases in total 15N loss, and 32.3–84.7 % reductions in NH3 volatilization compared with the conventional urea. These results provide strong evidence that substituting a basal polymer-coated urea application for three split urea applications is a viable alternative for improving N use efficiency and reducing N pollution in the intensive rice agroecosystem of southern China. However, improved N use efficiency by polymer-coated urea did not consistently lead to increases in rice yield. The results from the pot experiment examining 15N release from the three types of polymer-coated urea and 15N uptake by rice plants imply that avoiding large N releases in the rice seedling stage, 6 % polymer-coated urea for example, and in the rice ripening stage, 8 % polymer-coated urea for example, should be better for a maximally positive yield response. Although the 12 % polymer-coated urea treatment used here had a consistently higher N use efficiency and a greater rice yield compared with the other two, it should be noted that N release from 12 % polymer-coated urea did not correspond well with the plant demand for N at rice tillering and heading. Because soil temperature and moisture have less seasonal and yearly variability for mostly flooded summer rice in the subtropics of southern China than other agroecosystems, we believe that good synchronization of N release from polymer-coated urea with rice plant N demand can be accomplished by formulation alternation (e.g., varying the coating thickness, polymer materials, or polymer-coated urea blends), and thereafter achieve a significant improvement in rice production potential. Further long-term studies should focus on the optimum polymer-coated urea type and application rate in the rice ecosystems of the Taihu Lake region in southern China, taking into account both economic considerations and the environment.
References
Aarnio T, Martikainen PJ (1995) Mineralization of C and N and nitrification in Scots pine forest soil treated with nitrogen fertilizers containing different proportions of urea and its slow-releasing derivative, urea-formaldehyde. Soil Biol Biochem 27:1325–1331. doi:10.1016/0038-0717(95)00066-N
Cai GX, Freney JR, Humphreys E, Demand OT, Samson M, Simpson JR (1988) Use of surface film to reduce NH3 volatilization from flooded rice fields. Aust J Agric Res 39:177–186. doi:10.1071/AR9880177
Cheng DD, Zhang M, Yang YC, Liu M, Liu ZG (2011) Effects of controlled-release fertilizer residual coating on properties of soil and growth of winter wheat and summer corn. J Soil Water Conserv 25:225–235
Chien SH, Prochnow LI, Cantarella H (2009) Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv Agron 102:267–322. doi:10.1016/S0065-2113(09)01008-6
Food and Agriculture Organization of the United Nations (FAO) (2008) Current world fertilizer trends and outlook to 2011/12. Rome
Galloway JN, Townsend AR, Erisman JW, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892. doi:10.1126/science.1136674
Inubushi K, Acquaye S, Tsukagoshi S, Shibahara F, Komatsu S (2002) Effects of controlled-release coated urea (CRCU) on soil microbial biomass N in paddy fields examined by the 15N tracer technique. Nutr Cycl Agroecosyst 63:291–300. doi:10.1023/A:1021111126976
Kamekawa KI, Nagai T, Sekiya SI, Yoneyama T (1990) Nitrogen uptake by paddy rice (Oryza sativa L.) from 15N labeled coated urea and ammonium sulfate. Soil Sci Plant Nutr 36:333–336. doi:10.1080/00380768.1990.10414999
Kissel DE, Brewer HL, Arkin GF (1977) Design and test of a field sampler for ammonia volatilization. Soil Sci Soc Am J 41:1133–1138. doi:10.2136/sssaj1977.0361599500410 0060024x
Kochba M, Gambash S, Avnimelech Y (1990) Studies on slow release fertilizers: I. Effects of temperature, soil moisture, and water vapor pressure. Soil Sci 149:339–343. doi:10.1097/00010694-199006000-00004
Lin B, Li JK (2007) Effect evaluation of polymer-coated urea from Shandong Kingenta Ecological Engineering Co. Ltd. China Agri Technol Ext 23:42–43
Lu RK (2000) Soil agro-chemical analyses. Agri Tech Press, Bei**g
National Bureau of Statistics of China (2011) China statistical year 2011, chapter 13-6: irrigated area, consumption of chemical fertilizers and rural hydropower stations and electricity consumption in rural areas. http://www.stats.gov.cn/tjsj/ndsj/2011/html /M1306e.htm. China Stat. Press, Bei**g
Ning TY, Shao GQ, Li ZJ, Han HF, Hu HG, Wang Y, Tian SZ, Chi SY (2012) Effects of urea types and irrigation on crop uptake, soil residual, and loss of nitrogen in maize field on the North China Plain. Plant Soil Environ 58:1–8
Shaviv A (2001) Advances in controlled-release fertilizers. Adv Agron 71:1–49. doi:10.1016/S0065-2113(01)71011-5
Shoji S (1999) Meister controlled release rertilizer. Konno Printing Company Ltd, Sendai
Smil V (1999) Detonator of the population explosion. Nature 400:415. doi:10.1038/22672
Trenkel ME (1997) Controlled-release and stabilized fertilizers in agriculture, vol 11. International Fertilizer Industry Association, Paris
Wada G, Shoji S, Mae T (1986) Relationship between nitrogen absorption and growth and yield of rice plants. JPN Agric Res Q 20:135–145
Watson C (2013) Slow and controlled release and stabilized fertilizers: a growing market. New Ag Int 9:33–35
Wilson ML, Rosen CJ, Moncrief JF (2009) A comparison of techniques for determining nitrogen release from polymer-coated urea in the field. HortSci 44:492–494
Yang YC, Zhang M, Zheng L, Cheng DD, Liu M, Geng YQ (2011) Controlled release urea improved nitrogen use efficiency, yield and quality of wheat. Agron J 103:479–485. doi:10.2134/agronj2010.0343
Yang YC, Zhang M, Li YC, Fan XH, Geng YQ (2012a) Improving the quality of polymer-coated urea with recycled plastic, proper additives, and large tablets. J Agric Food Chem 60:11229–11237. doi:10.1021/jf302813g
Yang YC, Zhang M, Li YC, Fan XH, Geng YQ (2012b) Controlled release urea improved nitrogen use efficiency, activities of leaf enzymes, and rice yield. Soil Sci Soc Am J 76:2307–2317. doi:10.2136/sssaj2012.0173
Yang YC, Zhang M, Zheng L, Cheng DD, Liu M, Geng YQ (2013) Controlled release urea for rice production and its environmental implications. J Plant Nutri 36:781–794. doi:10.1080/01904167.2012.756892
Zhang M, Yang YC, Wan LB (2006) Production method of controlled release fertilizer by use recycled thermoplastic resin. Chinese Patent ZL.2004100357833. Date issued: 24 May. Shandong Agriculture University and Kingenta Ecological Engineering Co. Ltd
Zhang WJ, Li GH, Yang YM, Li Q, Zhang J, Liu JY, Wang SH, Tang S, Ding YF (2013) Effects of nitrogen application rate and ratio on lodging resistance of super rice with different genotypes. J Integr Agri 13:63–72. doi:10.1016/S2095-3119(13)60388-3
Zhao X, Zhou Y, Wang SQ, ** system in southern China. Soil Sci Soc Am J 76:1068–1078. doi:10.2136/sssaj2011.0236
Zhu ZL, Chen DL (2002) Nitrogen fertilizer use in China-contribution to food production, impacts on the environment and best management strategies. Nutr Cycl Agroecosys 63:117–127. doi:10.1023/A:1021107026067
Zhu ZL, Wen QX, Freney JR (1997) Nitrogen in soils of China. Kluwer Academic Publishers, Dordrecht
Acknowledgments
This study was funded by the National Key Technology R&D Program of China (2011BAD11B01; 2012BAD15B03) and the Special S&T Project on Treatment and Control of Water Pollution (2012ZX07101-004). We deeply appreciate anonymous reviewers and editors for their valuable suggestions that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wang, S., Zhao, X., **ng, G. et al. Improving grain yield and reducing N loss using polymer-coated urea in southeast China. Agron. Sustain. Dev. 35, 1103–1115 (2015). https://doi.org/10.1007/s13593-015-0300-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13593-015-0300-7