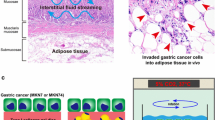
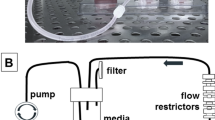
Abstract
The importance of the microenvironment is widely recognized as it regulates not only malignant cell behavior but also drug sensitivity. The cancer cell microenvironment is composed of biological, physical and chemical elements, and simultaneous reproduction of these three elements are important conditions investigated in cancer research. In the present study, we focused on the epidemiological and anatomical specificities of endometrioid carcinoma, obesity (biological), fluid flow (physical) and anticancer agents (chemical) to target the specific microenvironmental elements of endometrioid carcinoma. To elucidate the individual effects of these elements on endometrioid carcinoma and to investigate the relationships between these factors, we developed an adipose tissue fragments (ATFs)-embedded cell disc under a rotational culture method to generate carcinoma-stroma interactions and to create fluid flow. ATFs and fluid flow individually or synergistically influenced proliferative cellular behavior and the morphological changes underlying endometrioid carcinoma. ATFs and fluid flow also governed the expression of extracellular signal-regulated kinase and p38 signaling synergistically or individually, depending on the endometrioid carcinoma cell type. Adipose tissue induced chemoresistance to cis-diamminedichloro-platinum (CDDP) in endometrioid cancer, but the resistance effect was abolished by fluid flow. Thus, a simple reconstructed model was established to investigate three elements of the microenvironment of endometrioid carcinoma in vitro. This culture model unequivocally demonstrated the individual and synergistic effects of the three elements on endometrioid carcinoma. This new culture model is a promising tool for elucidating the mechanisms underlying endometrioid carcinoma and for develo** further treatment strategies.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
World Health Organization. Female genital tumours 5th edn. Lyon, France. International Agency for Research on Cancer. Geneva, Switzerland: Print copies are distributed by WHO Press; 2020.
Clement PB, Young RH. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2002;9:145–84.
Nevadunsky NS, Van Arsdale A, Strickler HD, et al. Obesity and age at diagnosis of endometrial cancer. Obstet Gynecol. 2014;124:300–6.
Feng Y-H. The association between obesity and gynecological cancer. Gynecol Minim Invasive Ther. 2015;4:102–5.
Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48: e12997.
Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des. 2010;16:1896–901.
Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7:57–62.
Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Bustos M, Martínez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta (BBA) Bioenerg. 2011;1807:664–78.
Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–6.
Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–66.
Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–8.
Senthebane DA, Rowe A, Thomford NE, et al. The role of tumor microenvironment in chemoresistance: to survive, keep your enemies closer. Int J Mol Sci. 2017;18:1586.
Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol. 2018;6:18.
Komohara Y, Takeya M. CAFs and TAMs: maestros of the tumour microenvironment. J Pathol. 2017;241:313–5.
Mitchell MJ, King MR. Computational and experimental models of cancer cell response to fluid shear stress. Front Oncol. 2013;3:44.
Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res. 2013;1:210–6.
Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–54.
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185.
Matsumoto M, Yamaguchi Y, Seino Y, et al. Estrogen signaling ability in human endometrial cancer through the cancer–stromal interaction. Endocr Relat Cancer. 2008;15:451–63.
Arnold JT, Lessey BA, Seppälä M, Kaufman DG. Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells. Can Res. 2002;62:79–88.
Mescher AL. Junqueira’s basic histology: text and atlas, Sixteenth edition, 50th anniversary edition. New York: McGraw-Hill; 2021.
Kim SW, Ehrman J, Ahn MR, et al. Shear stress induces noncanonical autophagy in intestinal epithelial monolayers. Mol Biol Cell. 2017;28:3043–56.
Lai SK, Wang Y-Y, Wirtz D, Hanes J. Micro-and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61:86–100.
Van Wijk F, Van der Burg M, Burger CW, Vergote I, van Doorn HC. Management of recurrent endometrioid endometrial carcinoma: an overview. Int J Gynecol Cancer. 2009;19:314–20.
Nagase K, Akutagawa T, Rikitake-Yamamoto M, et al. Cellular and physical microenvironments regulate the aggressiveness and sunitinib chemosensitivity of clear cell renal cell carcinoma. J Pathol. 2021;254:46–56.
Aoki S, Toda S, Ando T, Sugihara H. Bone marrow stromal cells, preadipocytes, and dermal fibroblasts promote epidermal regeneration in their distinctive fashions. Mol Biol Cell. 2004;15:4647–57.
Aoki S, Makino J, Nagashima A, et al. Fluid flow stress affects peritoneal cell kinetics: possible pathogenesis of peritoneal fibrosis. Perit Dial Int. 2011;31:466–76.
Donohoe F, Wilkinson M, Baxter E, Brennan DJ. Mitogen-activated protein kinase (MAPK) and obesity-related cancer. Int J Mol Sci. 2020;21:1241.
Wilson J, Balkwill F. The role of cytokines in the epithelial cancer microenvironment. In: Seminars in cancer biology. Elsevier; 2002. p. 113–20.
Kawata K, Aoki S, Futamata M, et al. Mesenchymal cells and fluid flow stimulation synergistically regulate the kinetics of corneal epithelial cells at the air–liquid interface. Graefes Arch Clin Exp Ophthalmol. 2019;257:1915–24.
Makker A, Goel MM, Das V, Agarwal A. PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: an update. Gynecol Endocrinol. 2012;28:175–81.
Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev. 2011;12:1063–70.
Liu F, Yang X, Geng M, Huang M. Targeting ERK, an Achilles’ Heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B. 2018;8:552–62.
Housman G, Byler S, Heerboth S, et al. Drug resistance in cancer: an overview. Cancers. 2014;6:1769–92.
Tsuruo T, Naito M, Tomida A, et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94:15–21.
McCubrey JA, Steelman LS, Kempf CR, et al. Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J Cell Physiol. 2011;226:2762–81.
Ramakrishnan VM, Boyd NL. The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Eng Part B Rev. 2018;24:289–99.
Wang T, Sharma AK, Wolfrum C. Novel insights into adipose tissue heterogeneity. Rev Endocr Metab Disord. 2022;23:5–12.
Lihn A, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21.
Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809.
Aoki S, Toda S, Sakemi T, Sugihara H. Coculture of endothelial cells and mature adipocytes actively promotes immature preadipocyte development in vitro. Cell Struct Funct. 2003;28:55–60.
Acknowledgements
We thank S. Nishimura, M. Nishida and S. Nakahara for excellent technical assistance.
Funding
This work was partially supported by grants from JSPS KAKENHI Grant Number 19K18468 (to MN) and 21K16773 (to MH).
Author information
Authors and Affiliations
Contributions
SM: Investigation, Writing—Original Draft. MK Investigation, Methodology. MN, TS, MH, TN: Investigation. AK: Statistical analysis. ST: Supervision. SA: Conceptualization, Methodology, Investigation, Writing—Reviewing and Editing, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures involving human or animal materials were performed in accordance with the ethical guidelines of Saga University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morito, S., Kawasaki, M., Nishiyama, M. et al. Microenvironmental elements singularity synergistically regulate the behavior and chemosensitivity of endometrioid carcinoma. Human Cell 36, 1147–1159 (2023). https://doi.org/10.1007/s13577-023-00886-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00886-7