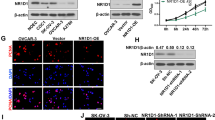
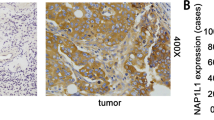
Abstract
Non-catalytic region of tyrosine kinase adaptor protein 1 (Nck1) is crucial for the progression of cancers. However, little is known on the role of Nck1 in the progression of ovarian carcinoma (OC). Here, we show that Nck1 expression is up-regulated in 176 OC tissues, compared with non-carcinoma ovarian tissues, and the up-regulated Nck1 expression is associated with the aggressiveness of OC and shorter overall and disease-free survival in this population. Higher Nck1 expression was an independent risk factor for poor prognosis of OC. Furthermore, Nck1 silencing by short hairpin RNA (shRNA) technology significantly inhibited the proliferation, migration and invasion of OC cells in vitro and the growth and metastasis of implanted OC tumors in vivo. Human kinase phosphorylation array indicated that Nck1 silencing significantly reduced the relative levels of 11 kinase expression and phosphorylation in OC cells, particularly for decreased levels of p70S6 kinase (p70S6K) and protein kinase B (AKT) expression in SKOV3 cells. Actually, Nck1 silencing significantly decreased PI3K and AKT expression, and reduced AKT and p70S6K phosphorylation while Nck1 over-expression had opposite effects in OC cells. Therefore, our data indicate that Nck1 promotes the progression of OC by enhancing the PI3k/AKT/p70S6K signaling in OC. Our findings suggest that Nck1 expression may be valuable for evaluating the prognosis of OC and as a target for design of new therapies for OC.




Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Peres LC, Cushing-Haugen KL, Kobel M, Harris HR, Berchuck A, Rossing MA, et al. Invasive epithelial ovarian cancer survival by histotype and disease stage. J Natl Cancer Inst. 2018. https://doi.org/10.1093/jnci/djy071.
Mills K, Fuh K. Recent advances in understanding, diagnosing, and treating ovarian cancer. F1000Research. 2017;6:84. https://doi.org/10.12688/f1000research.9977.1.
Buvall L, Rashmi P, Lopez-Rivera E, Andreeva S, Weins A, Wallentin H, et al. Proteasomal degradation of Nck1 but not Nck2 regulates RhoA activation and actin dynamics. Nat Commun. 2013;4:2863. https://doi.org/10.1038/ncomms3863.
Ngoenkam J, Paensuwan P, Preechanukul K, Khamsri B, Yiemwattana I, Beck-Garcia E, et al. Non-overlap** functions of Nck1 and Nck2 adaptor proteins in T cell activation. Cell Commun Signal CCS. 2014;12:21. https://doi.org/10.1186/1478-811x-12-21.
Buday L, Wunderlich L, Tamas P. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal. 2002;14(9):723–31.
Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–59. https://doi.org/10.1146/annurev.immunol.26.021607.090347.
Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418(6899):790–3. https://doi.org/10.1038/nature00859.
Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem. 2001;276(28):26448–52. https://doi.org/10.1074/jbc.M103856200.
Ditlev JA, Michalski PJ, Huber G, Rivera GM, Mohler WA, Loew LM, et al. Stoichiometry of Nck-dependent actin polymerization in living cells. J Cell Biol. 2012;197(5):643–58. https://doi.org/10.1083/jcb.201111113.
Abella JV, Vaillancourt R, Frigault MM, Ponzo MG, Zuo D, Sangwan V, et al. The Gab1 scaffold regulates RTK-dependent dorsal ruffle formation through the adaptor Nck. J Cell Sci. 2010;123(Pt 8):1306–19. https://doi.org/10.1242/jcs.062570.
Qiu F, Huang D, **ao H, Qiu F, Lu L, Nie J. Detection of tyrosinephosphorylated proteins in hepatocellular carcinoma tissues using a combination of GSTNck1SH2 pulldown and two dimensional electrophoresis. Mol Med Rep. 2013;7(4):1209–14. https://doi.org/10.3892/mmr.2013.1324.
Paensuwan P, Hartl FA, Yousefi OS, Ngoenkam J, Wipa P, Beck-Garcia E, et al. Nck binds to the T cell antigen receptor using its SH3.1 and SH2 domains in a cooperative manner, promoting TCR functioning. J Immunol (Baltimore, Md: 1950). 2016;196(1):448–58. https://doi.org/10.4049/jimmunol.1500958.
**a P, Huang M, Zhang Y, **ong X, Yan M, **ong X, et al. NCK1 promotes the angiogenesis of cervical squamous carcinoma via Rac1/PAK1/MMP2 signal pathway. Gynecol Oncol. 2019;152(2):387–95. https://doi.org/10.1016/j.ygyno.2018.11.013.
Zhang F, Lu YX, Chen Q, Zou HM, Zhang JM, Hu YH, et al. Identification of NCK1 as a novel downstream effector of STAT3 in colorectal cancer metastasis and angiogenesis. Cell Signal. 2017;36:67–78. https://doi.org/10.1016/j.cellsig.2017.04.020.
Morris DC, Popp JL, Tang LK, Gibbs HC, Schmitt E, Chaki SP, et al. Nck deficiency is associated with delayed breast carcinoma progression and reduced metastasis. Mol Biol Cell. 2017;28(24):3500–16. https://doi.org/10.1091/mbc.E17-02-0106.
Fanelli M, Camperchioli A, Petrella L, Petrillo M, Baranello C, Baccaro P, et al. Non-catalytic region of tyrosine kinase adaptor protein 2 (NCK2) pathways as factor promoting aggressiveness in ovarian cancer. The International journal of biological markers. 2018;33(1):124–31. https://doi.org/10.5301/ijbm.5000264.
Zhang J, Zhou S, Tang L, Shen L, **ao L, Duan Z, et al. WAVE1 gene silencing via RNA interference reduces ovarian cancer cell invasion, migration and proliferation. Gynecol Oncol. 2013;130(2):354–61. https://doi.org/10.1016/j.ygyno.2013.05.005.
Zhang J, Tang L, Shen L, Zhou S, Duan Z, **ao L, et al. High level of WAVE1 expression is associated with tumor aggressiveness and unfavorable prognosis of epithelial ovarian cancer. Gynecol Oncol. 2012;127(1):223–30. https://doi.org/10.1016/j.ygyno.2012.06.008.
Rosner M, Hengstschlager M. mTOR protein localization is cell cycle-regulated. Cell cycle (Georgetown, Tex). 2011;10(20):3608–10. https://doi.org/10.4161/cc.10.20.17855.
Griffin MJ. Synchronization of some human cell strains by serum and calcium starvation. vitro. 1976;12(5):393–8. https://doi.org/10.1007/bf02796317.
Mayer IA, Arteaga CL. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu Rev Med. 2016;67:11–28. https://doi.org/10.1146/annurev-med-062913-051343.
Nieto-Pelegrin E, Kenny B, Martinez-Quiles N. Nck adaptors, besides promoting N-WASP mediated actin-nucleation activity at pedestals, influence the cellular levels of enteropathogenic Escherichia coli Tir effector. Cell adhesion & migration. 2014;8(4):404–17. https://doi.org/10.4161/19336918.2014.969993.
Okrut J, Prakash S, Wu Q, Kelly MJ, Taunton J. Allosteric N-WASP activation by an inter-SH3 domain linker in Nck. Proc Natl Acad Sci USA. 2015;112(47):E6436–E64456445. https://doi.org/10.1073/pnas.1510876112.
Chaki SP, Rivera GM. Integration of signaling and cytoskeletal remodeling by Nck in directional cell migration. Bioarchitecture. 2013;3(3):57–63. https://doi.org/10.4161/bioa.25744.
Huang M, Anand S, Murphy EA, Desgrosellier JS, Stupack DG, Shattil SJ, et al. EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation. Oncogene. 2012;31(22):2783–93. https://doi.org/10.1038/onc.2011.450.
Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ, et al. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 2010;123(Pt 21):3662–733. https://doi.org/10.1242/jcs.068163.
Guan T, Huang K, Liu Y, Hou S, Hu C, Li Y, et al. Aristolochic acid inhibits Slit2-induced migration and tube formation via inactivation of Robo1/Robo2-NCK1/NCK2 signaling pathway in human umbilical vein endothelial cells. Toxicol Lett. 2019;300:51–8. https://doi.org/10.1016/j.toxlet.2018.10.022.
Li W, She H. The SH2 and SH3 adapter Nck: a two-gene family and a linker between tyrosine kinases and multiple signaling networks. Histol Histopathol. 2000;15(3):947–55. https://doi.org/10.14670/HH-15.947.
Hu Q, Milfay D, Williams LT. Binding of NCK to SOS and activation of ras-dependent gene expression. Mol Cell Biol. 1995;15(3):1169–74. https://doi.org/10.1128/mcb.15.3.1169.
Rajalingam K, Schreck R, Rapp UR, Albert S. Ras oncogenes and their downstream targets. Biochem Biophys Acta. 2007;1773(8):1177–95. https://doi.org/10.1016/j.bbamcr.2007.01.012.
Qian Y, Corum L, Meng Q, Blenis J, Zheng JZ, Shi X, et al. PI3K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am J Physiol Cell Physiol. 2004;286(1):C153–C163163. https://doi.org/10.1152/ajpcell.00142.2003.
Previs RA, Armaiz-Pena GN, Ivan C, Dalton HJ, Rupaimoole R, Hansen JM, et al. Role of YAP1 as a marker of sensitivity to dual AKT and P70S6K inhibition in ovarian and uterine malignancies. J Natl Cancer Inst. 2017. https://doi.org/10.1093/jnci/djw296.
Acknowledgements
The authors thank the Laboratory Research Center, the First Affiliated Hospital of Chongqing Medical University for their experimental supplies.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (Grant No. 81372800).
Author information
Authors and Affiliations
Contributions
XL, JZ and LT designed the experiments. MH collected tissue samples. XL, JZ, ZD, XF and YY carried out the experiments. XL and XF analyzed the experimental results. XL and LT wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and informed consent
The human and animal experimental protocols were approved by the Ethical Review Board of the First Affiliated Hospital of Chongqing Medical University, Chongqing, China (No. 20181601). Written informed consent in accordance with the Declaration of Helsinki was obtained from all the enrolled patients for the use of their tissue specimens. All experiments on animals followed the Laboratory Animal Guideline.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, X., Zhang, J., Duan, Z. et al. Nck1 promotes the progression of ovarian carcinoma by enhancing the PI3K/AKT/p70S6K signaling. Human Cell 33, 768–779 (2020). https://doi.org/10.1007/s13577-020-00344-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00344-8