Abstract
Objective
This study aimed to investigate the concentration of Wingless-type (Wnt)-inducible signaling pathway protein-1 (WISP1) in the serum, and its expression in the abdominal subcutaneous adipose tissue (SAT), and placenta of women with gestational diabetes mellitus (GDM).
Methods
A total of 69 patients with GDM and 71 pregnant women with normal glucose tolerance (NGT, control) were recruited. Carbohydrate metabolism, alanine aminotransferase (ALT), lipid profiles, thyroid function, interleukin-6 (IL-6), and WISP1 levels were assessed. Fasting sera were collected between 25 and 30 weeks of gestation. Tissues of placenta and abdominal SAT samples were obtained from 24 women who had undergone cesarean section and were divided into a GDM group and a control group. Reverse transcription polymerase chain reaction (RT-PCR) and western blot were used to detect the WISP1 expression.
Results
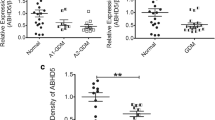
The serum WISP1 concentrations were higher in the GDM group than in the control group (p < 0.01) and positively associated with body mass index (BMI), fasting glucose, fasting insulin, HOMA-insulin resistance (HOMA-IR), and IL-6 levels. BMI, fasting glucose, and HOMA-IR independently and positively predicted WISP1 levels. Further, WISP1 mRNA and protein expression were higher in tissues from the placenta and abdominal SAT from the GDM group (p < 0.01).
Conclusions
WISP1 may be an important adipokine in modulating carbohydrate metabolism in women with GDM.



Similar content being viewed by others
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
References
Association AD. Classification and diagnosis of diabetes. Diabetes Care. 2016;39(Suppl 1):S13-22.
Poulakos P, Mintziori G, Tsirou E, Taousani E, Savvaki D, Harizopoulou V, et al. Comments on gestational diabetes mellitus: from pathophysiology to clinical practice. Hormones (Athens, Greece). 2015;14(3):335–44.
Sook LW, Sablihan NI, Ismail S, Devaraj NK, Mooi CS. Factors associated with the level of physical activities among non-academic staffs in the faculty of medicine and health sciences of a public university in Selangor. Malaysia Mal J Med Health Sci. 2019;15(2):47–55.
Lee KW, Ching SM, Hoo FK, Ramachandran V, Chong SC, Tusimin M, et al. Neonatal outcomes and its association among gestational diabetes mellitus with and without depression, anxiety and stress symptoms in Malaysia: a cross-sectional study. Midwifery. 2020;81:102586.
Devaraj NK, Mohamed M, Hussein N. Prevalence, factors influencing and knowledge about adherence to lipid-lowering therapy among hyperlipidemia patients. Med J Malaysia. 2017;72(3):157–64.
Kintiraki E, Goulis DG, Mameletzi S, Kasmas S, Athanasiadis A, Assimakopoulos E, et al. Large- and small-for-gestational-age neonates born by women with gestational diabetes mellitus diagnosed by the new IADPSG criteria: a case-control study of 289 patients and 1108 controls. Exp Clin Endocrinol Diabetes. 2013;121(5):262–5.
Stuebe AM, Landon MB, Lai Y, Spong CY, Carpenter MW, Ramin SM, et al. Maternal BMI, glucose tolerance, and adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;207(1):62.e1-7.
Tsiotra PC, Halvatsiotis P, Patsouras K, Maratou E, Salamalekis G, Raptis SA, et al. Circulating adipokines and mRNA expression in adipose tissue and the placenta in women with gestational diabetes mellitus. Peptides. 2018;101:157–66.
Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103–13.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97.
Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–223.
Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27(8):794–8.
Aye IL, Lager S, Ramirez VI, Gaccioli F, Dudley DJ, Jansson T, et al. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod. 2014;90(6):129.
Kleiblova P, Dostalova I, Bartlova M, Lacinova Z, Ticha I, Krejci V, et al. Expression of adipokines and estrogen receptors in adipose tissue and placenta of patients with gestational diabetes mellitus. Mol Cell Endocrinol. 2010;314(1):150–6.
Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, et al. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98(1):105–13.
Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Docke S, Keyhani-Nejad F, et al. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes. 2015;64(3):856–66.
Barchetta I, Cimini FA, Capoccia D, De Gioannis R, Porzia A, Mainiero F, et al. WISP1 is a marker of systemic and adipose tissue inflammation in dysmetabolic subjects with or without type 2 diabetes. J Endocr Soc. 2017;1(6):660–70.
Wang AR, Yan XQ, Zhang C, Du CQ, Long WJ, Zhan D, et al. Characterization of Wnt1-inducible signaling pathway protein-1 in obese children and adolescents. Curr Med Sci. 2018;38(5):868–74.
Jung TW, Kang C, Goh J, Chae SI, Kim HC, Lee TJ, et al. WISP1 promotes non-alcoholic fatty liver disease and skeletal muscle insulin resistance via TLR4/JNK signaling. J Cell Physiol. 2018;233(8):6077–87.
Maiese K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr Neurovasc Res. 2014;11(4):378–89.
Association AD. Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl 1):S11-61.
Palsgaard J, Emanuelli B, Winnay JN, Sumara G, Karsenty G, Kahn CR. Cross-talk between insulin and Wnt signaling in preadipocytes: role of Wnt co-receptor low density lipoprotein receptor-related protein-5 (LRP5). J Biol Chem. 2012;287(15):12016–26.
Liu L, Hu J, Yang L, Wang N, Liu Y, Wei X. Association of WISP1/CCN4 with Risk of overweight and gestational diabetes mellitus in Chinese pregnant women. Dis Markers. 2020;2020:4934206.
Sahin Ersoy G, Altun Ensari T, Subas S, Giray B, Simsek EE, Cevik O. WISP1 is a novel adipokine linked to metabolic parameters in gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2017;30(8):942–6.
Morrison MC, Kleemann R. Role of macrophage migration inhibitory factor in obesity, insulin resistance, type 2 diabetes, and associated hepatic co-morbidities: a comprehensive review of human and rodent studies. Front Immunol. 2015;6:308.
Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59(6):1075–88.
Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010;22(5):809–20.
Wang S, Chong ZZ, Shang YC, Maiese K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012;9(1):20–31.
Lim HW, Lee JE, Shin SJ, Lee YE, Oh SH, Park JY, et al. Identification of differentially expressed mRNA during pancreas regeneration of rat by mRNA differential display. Biochem Biophys Res Commun. 2002;299(5):806–12.
Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr Neurovasc Res. 2013;10(1):54–69.
Campos DB, Palin MF, Bordignon V, Murphy BD. The “beneficial” adipokines in reproduction and fertility. Int J Obes (Lond). 2008;32(2):223–31.
Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–81.
Kuzmicki M, Telejko B, Wawrusiewicz-Kurylonek N, Citko A, Lipinska D, Pliszka J, et al. The expression of suppressor of cytokine signaling 1 and 3 in fat and placental tissue from women with gestational diabetes. Gynecol Endocrinol. 2012;28(11):841–4.
Lepercq J, Cauzac M, Lahlou N, Timsit J, Girard J, Auwerx J, et al. Overexpression of placental leptin in diabetic pregnancy: a critical role for insulin. Diabetes. 1998;47(5):847–50.
Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36(7):709–15.
Segovia SA, Vickers MH, Gray C, Reynolds CM. Maternal obesity, inflammation, and developmental programming. Biomed Res Int. 2014;2014:418975.
Strakovsky RS, Pan YX. A decrease in DKK1, a WNT inhibitor, contributes to placental lipid accumulation in an obesity-prone rat model. Biol Reprod. 2012;86(3):81.
Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin Ther Targets. 2012;16(12):1203–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Human Ethics Committee of the Sheng**g Hospital of China Medical University. It was designed in accordance with the principle of the Helsinki Declaration.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Lc., Xu, St. & Li, L. WISP1 is increased in the maternal serum, adipose tissue, and placenta of women with gestational diabetes mellitus. Int J Diabetes Dev Ctries 42, 269–275 (2022). https://doi.org/10.1007/s13410-021-00972-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-021-00972-2