Abstract
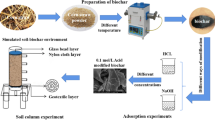
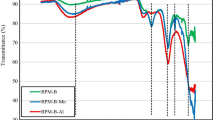
Biochar has great potential for climate change mitigation and heavy metal pollution remediation, but is susceptible to aging and expensive to prepare. The aim of this study was to produce biochar materials with long-term stability and cost-effective for heavy metal removal. The physicochemical properties, stability, adsorption performance, and cost-effectiveness of biochar were compared using co-pyrolysis of rice husk (Oryza sativa L.), mulberry twigs (Morus alba L.), and reeds (Phragmites australis (Cav.) Trin. ex Steud) with vermiculite at 500 ℃. The results showed that minerals would cover the surface of the biochar while carbothermal reduction reaction occurred to form a stable Si-C system, which enhanced the chemical, thermal, and biochar stability of vermiculite-modified biochar. Both vermiculite-modified biochar enhanced the adsorption capacity and adsorption stability of Hg, and the kinetic adsorption characteristics of Hg before and after biochar modification were consistent with the quasi-secondary kinetic model, and the isothermal adsorption characteristics were more consistent with the Langmuir model. Vermiculite-modified mulberry twig biochar (W-SBC) had the highest removal efficiency (93.48%) due to its large specific surface area and pore structure, with a maximum adsorption capacity of 67.551 mg·g−1, and adsorption was dominated by the complexation of -OH and C = O. Economic analysis showed that vermiculite-modified rice husk biochar (W-DBC) had the highest cost-effectiveness with a removal cost of 1.51 USD·g−1 Hg. By weighing carbon stability and cost-effectiveness, W-DBC can be used as a cost-effective material to enhance the potential of carbon sequestration and application for Hg pollution remediation.











Similar content being viewed by others
Data availability
Data from this study is available on request from the corresponding author.
References
IPCC (2018) Global warming of 1.5°C: An IPCC special report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. In: Masson-Delmotte V, Zhai P, Pörtner H-O, Roberts D, Skea J, Shukla PR, Pirani A, Moufouma-Okia W, Péan C, Pidcock R, Connors S, Matthews JBR, Chen Y, Zhou X, Gomis MI, Lonnoy E, Maycock T, Tignor M, Waterfield T (eds). Cambridge University Press. https://doi.org/10.1017/9781009157940/
Mora C, Spirandelli D, Franklin EC et al (2018) Broad threat to humanity from cumulative climate hazards intensified by greenhouse gas emissions. Nat Clim Change 8:1062–1071. https://doi.org/10.1038/s41558-018-0315-6
Zong Y, **ao Q, Lu S (2021) Biochar derived from cadmium-contaminated rice straw at various pyrolysis temperatures: cadmium immobilization mechanisms and environmental implication. Bioresour Technol 321:124459. https://doi.org/10.1016/j.biortech.2020.124459
Liu Y, Lu H, Yang S, Wang Y (2016) Impacts of biochar addition on rice yield and soil properties in a cold waterlogged paddy for two crop seasons. Field Crops Res 191:161–167. https://doi.org/10.1016/j.fcr.2016.03.003
Tan G, Sun W, Xu Y et al (2016) Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour Technol 211:727–735. https://doi.org/10.1016/j.biortech.2016.03.147
Wang F, Zhang R, Donne SW et al (2022) Co-pyrolysis of wood chips and bentonite/kaolin: influence of temperatures and minerals on characteristics and carbon sequestration potential of biochar. Sci Total Environ 838:156081. https://doi.org/10.1016/j.scitotenv.2022.156081
Han L, Ro KS, Wang Y et al (2018) Oxidation resistance of biochars as a function of feedstock and pyrolysis condition. Sci Total Environ 616–617:335–344. https://doi.org/10.1016/j.scitotenv.2017.11.014
Zhao W, Zhang Z, **n Y et al (2024) Na2S-modified biochar for hg(II) removal from wastewater: a techno-economic assessment. Fuel 356:129641. https://doi.org/10.1016/j.fuel.2023.129641
Zhang B, Gao B, Ma W et al (2023) Adsorption of uranium (VI) by natural vermiculite: isotherms, kinetic, thermodynamic and mechanism studies. J Environ Radioact 270:107305. https://doi.org/10.1016/j.jenvrad.2023.107305
Abollino O, Giacomino A, Malandrino M, Mentasti E (2008) Interaction of metal ions with montmorillonite and vermiculite. Appl Clay Sci 38:227–236. https://doi.org/10.1016/j.clay.2007.04.002
Liu Y, Wang R, He L et al (2023) Microbial resistance stability of rice straw biochar with vermiculite modification: a novel insight into persistent free radicals and pore structure. J Environ Chem Eng 11:110572. https://doi.org/10.1016/j.jece.2023.110572
Kadam KL, Forrest LH, Jacobson WA (2000) Rice straw as a lignocellulosic resource: collection, processing, transportation, and environmental aspects. Biomass Bioenergy. https://doi.org/10.1016/S0961-9534(00)00005-2
Nguyen T-AD, Kim K-R, Han SJ et al (2010) Pretreatment of rice straw with ammonia and ionic liquid for lignocellulose conversion to fermentable sugars. Bioresour Technol 101:7432–7438. https://doi.org/10.1016/j.biortech.2010.04.053
Li R, Fei J, Cai Y et al (2009) Cellulose whiskers extracted from mulberry: a novel biomass production. Carbohydr Polym 76:94–99. https://doi.org/10.1016/j.carbpol.2008.09.034
Guo Y, Chen J-Q, Su M, Hong J-G (2018) Bio-based plastics with highly efficient esterification of lignocellulosic biomass in 1-methylimidazole under mild conditions. J Wood Chem Technol 38:338–349. https://doi.org/10.1080/02773813.2018.1488876
Prithivirajan R, Balasundar P, Shyamkumar R et al (2019) Characterization of cellulosic fibers from Morus alba L. stem. J Nat Fibers 16:503–511. https://doi.org/10.1080/15440478.2018.1426079
Raud M, Tutt M, Olt J, Kikas T (2016) Dependence of the hydrolysis efficiency on the lignin content in lignocellulosic material. Int J Hydrog Energy 41:16338–16343. https://doi.org/10.1016/j.ijhydene.2016.03.190
Jung S-J, Kim S-H, Chung I-M (2015) Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy 83:322–327. https://doi.org/10.1016/j.biombioe.2015.10.007
Li F, Cao X, Zhao L et al (2014) Effects of mineral additives on biochar formation: carbon retention, stability, and properties. Environ Sci Technol 48:11211–11217. https://doi.org/10.1021/es501885n
Liu Y, Gao C, Wang Y et al (2020) Vermiculite modification increases carbon retention and stability of rice straw biochar at different carbonization temperatures. J Clean Prod 254:120111. https://doi.org/10.1016/j.jclepro.2020.120111
Zhao Z, Nie T, Zhou W (2019) Enhanced biochar stabilities and adsorption properties for tetracycline by synthesizing silica-composited biochar. Environ Pollut 254:113015. https://doi.org/10.1016/j.envpol.2019.113015
Leng L, Huang H, Li H et al (2019) Biochar stability assessment methods: a review. Sci Total Environ 647:210–222. https://doi.org/10.1016/j.scitotenv.2018.07.402
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301. https://doi.org/10.1021/es903140c
Zhou Y, Liu X, **ang Y et al (2017) Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: adsorption mechanism and modelling. Bioresour Technol 245:266–273. https://doi.org/10.1016/j.biortech.2017.08.178
Sun L, Chen D, Wan S, Yu Z (2015) Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour Technol 198:300–308. https://doi.org/10.1016/j.biortech.2015.09.026
Yang Y, Lin X, Wei B et al (2014) Evaluation of adsorption potential of bamboo biochar for metal-complex dye: equilibrium, kinetics and artificial neural network modeling. Int J Environ Sci Technol 11:1093–1100. https://doi.org/10.1007/s13762-013-0306-0
Shukla N, Sahoo D, Remya N (2019) Biochar from microwave pyrolysis of rice husk for tertiary wastewater treatment and soil nourishment. J Clean Prod 235:1073–1079. https://doi.org/10.1016/j.jclepro.2019.07.042
Demirbas A (2004) Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J Anal Appl Pyrol 72:243–248. https://doi.org/10.1016/j.jaap.2004.07.003
Shariff A, Aziz NSM, Saleh N, Ruzali NSI (2016) The effect of feedstock type and slow pyrolysis temperature on biochar yield from coconut wastes. Int J Chem Mol Eng 10:1410–1414
Zhang J, Liu J, Liu R (2015) Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour Technol 176:288–291. https://doi.org/10.1016/j.biortech.2014.11.011
Wang S, Gao B, Zimmerman AR et al (2015) Physicochemical and sorptive properties of biochars derived from woody and herbaceous biomass. Chemosphere 134:257–262. https://doi.org/10.1016/j.chemosphere.2015.04.062
Hedges JI, Eglinton G, Hatcher PG et al (2000) The molecularly-uncharacterized component of nonliving organic matter in natural environments. Org Geochem 31:945–958. https://doi.org/10.1016/S0146-6380(00)00096-6
Leng L, Huang H (2018) An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour Technol 270:627–642. https://doi.org/10.1016/j.biortech.2018.09.030
Tag AT, Duman G, Ucar S, Yanik J (2016) Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J Anal Appl Pyrol 120:200–206. https://doi.org/10.1016/j.jaap.2016.05.006
Inyang M, Gao B, Pullammanappallil P et al (2010) Biochar from anaerobically digested sugarcane bagasse. Bioresour Technol 101:8868–8872. https://doi.org/10.1016/j.biortech.2010.06.088
Ahmad M, Ahmad M, Usman ARA et al (2019) Date palm waste-derived biochar composites with silica and zeolite: synthesis, characterization and implication for carbon stability and recalcitrant potential. Environ Geochem Health 41:1687–1704. https://doi.org/10.1007/s10653-017-9947-0
Yang F, Xu Z, Huang Y et al (2021) Stabilization of dissolvable biochar by soil minerals: release reduction and organo-mineral complexes formation. J Hazard Mater 412:125213. https://doi.org/10.1016/j.jhazmat.2021.125213
Ippolito JA, Cui L, Kammann C et al (2020) Feedstock choice, pyrolysis temperature and type influence biochar characteristics: a comprehensive meta-data analysis review. Biochar 2:421–438. https://doi.org/10.1007/s42773-020-00067-x
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol 19:191–215. https://doi.org/10.1007/s11157-020-09523-3
Li M, Zhao Y, Ai Z et al (2021) Preparation and application of expanded and exfoliated vermiculite: a critical review. Chem Phys 550:111313. https://doi.org/10.1016/j.chemphys.2021.111313
Mikutta R, Kleber M, Kaiser K, Jahn R (2005) Rev Soil Sci Soc Am J 69:120. https://doi.org/10.2136/sssaj2005.0120
Zhao R, Jia L, Yao Y et al (2019) Study of the effect of adsorption temperature on elemental mercury removal performance of iron-based modified biochar. Energy Fuels 33:11408–11419. https://doi.org/10.1021/acs.energyfuels.9b02468
Lu J, Yang Y, Liu P et al (2020) Iron-montmorillonite treated corn straw biochar: interfacial chemical behavior and stability. Sci Total Environ 708:134773. https://doi.org/10.1016/j.scitotenv.2019.134773
Shaaban A, Se S-M, Mitan NMM, Dimin MF (2013) Characterization of biochar derived from rubber wood sawdust through slow pyrolysis on surface porosities and functional groups. Procedia Eng 68:365–371. https://doi.org/10.1016/j.proeng.2013.12.193
Harvey OR, Kuo L-J, Zimmerman AR et al (2012) An index-based approach to assessing recalcitrance and soil carbon sequestration potential of engineered black carbons (biochars). Environ Sci Technol 46:1415–1421. https://doi.org/10.1021/es2040398
Windeatt JH, Ross AB, Williams PT et al (2014) Characteristics of biochars from crop residues: potential for carbon sequestration and soil amendment. J Environ Manage 146:189–197. https://doi.org/10.1016/j.jenvman.2014.08.003
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253. https://doi.org/10.1021/es9031419
Deng B, Yuan X, Siemann E et al (2021) Feedstock particle size and pyrolysis temperature regulate effects of biochar on soil nitrous oxide and carbon dioxide emissions. Waste Manag 120:33–40. https://doi.org/10.1016/j.wasman.2020.11.015
Huang S, Liang Q, Geng J et al (2019) Sulfurized biochar prepared by simplified technic with superior adsorption property towards aqueous hg (II) and adsorption mechanisms. Mater Chem Phys 238:121919. https://doi.org/10.1016/j.matchemphys.2019.121919
Arias Arias FE, Beneduci A, Chidichimo F et al (2017) Study of the adsorption of mercury (II) on lignocellulosic materials under static and dynamic conditions. Chemosphere 180:11–23. https://doi.org/10.1016/j.chemosphere.2017.03.137
Zhu L, Tong L, Zhao N et al (2020) Key factors and microscopic mechanisms controlling adsorption of cadmium by surface oxidized and aminated biochars. J Hazard Mater 382:121002. https://doi.org/10.1016/j.jhazmat.2019.121002
Hou Y, Liang Y, Hu H et al (2021) Facile preparation of multi-porous biochar from lotus biomass for methyl orange removal: kinetics, isotherms, and regeneration studies. Bioresource Technol 329:124877. https://doi.org/10.1016/j.biortech.2021.124877
Pulido-Novicio L, Kurimoto Y, Aoyama M et al (2001) Adsorption of mercury by sugi wood carbonized at 1000~. J Wood Sci 47:159–162. https://doi.org/10.1007/BF00780567
Dong X, Ma LQ, Zhu Y et al (2013) Mechanistic investigation of mercury sorption by Brazilian pepper biochars of different pyrolytic temperatures based on X-ray photoelectron spectroscopy and flow calorimetry. Environ Sci Technol 47:12156–12164. https://doi.org/10.1021/es4017816
Bai Y, Hong J (2021) Preparation of a novel millet straw biochar-bentonite composite and its adsorption property of Hg2+ in aqueous solution. Materials 14:1117. https://doi.org/10.3390/ma14051117
Park J-H, Wang JJ, Zhou B et al (2019) Removing mercury from aqueous solution using sulfurized biochar and associated mechanisms. Environ Pollut 244:627–635. https://doi.org/10.1016/j.envpol.2018.10.069
Do Nascimento FH, Masini JC (2014) Influence of humic acid on adsorption of hg(II) by vermiculite. J Environ Manage 143:1–7. https://doi.org/10.1016/j.jenvman.2014.04.013
Tran L, Wu P, Zhu Y et al (2015) Comparative study of hg (II) adsorption by thiol- and hydroxyl-containing bifunctional montmorillonite and vermiculite. Appl Surf Sci 356:91–101. https://doi.org/10.1016/j.apsusc.2015.08.038
Funding
This research was funded by the funding project of Northeast Geological S&T Innovation Center of China Geological Survey (NO. QCJJ2022-25).
Author information
Authors and Affiliations
Contributions
Z. M.: conceptualization, data curation, investigation, methodology, writing (original draft); D. Z.: supervision, writing—review and editing; B. L.: visualization; H. L.: methodology. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This paper does not contain any human or animal studies.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Z., Zheng, D., Liang, B. et al. Effect of vermiculite-modified biochar on carbon sequestration potential, mercury adsorption stability, and economics. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05774-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05774-0