Abstract
Extracting and purifying lignin from wood without compromising cellulose quality is a challenging process. Lactic acid: choline chloride is a deep eutectic solvent (DES) that has been identified as acceptable delignification solvent, producing lignin and hemicellulose as byproducts to the cellulose. Hemicellulose is partly transformed into furanic compounds (such as furfural and 5-HMF). While the larger lignin can be obtained by water precipitation from DES, smaller lignin molecules and furanics can be recovered by liquid–liquid extraction (LLX), either directly or after precipitation of the larger lignin molecules. The presence of water in the DES after water precipitation reduced the mutual miscibility with the solvents, allowing the use of a wider range of solvents in the LLX process. In the precipitation step, all the larger molecular weight lignin (Mw > 5000 Da) can be recovered when adding at least 3.5:1 [g/g] water to DES-black liquor. For the LLX step, guaiacol was found as suitable alternative to the previously published 2-methyltetrahedrofuran (2-MTHF). In addition, here we report the use of 2,2,5,5-Tetramethyl oxolane (TMO), a recent addition to the palette of (potentially) bio-based solvents. The distribution coefficients of smaller lignin molecules and furanics in LLX with guaiacol, TMO and 2-MTHF were compared, revealing that smaller lignin molecules (500–5000 Da) can be recovered with a reasonable distribution coefficient by 2-MTHF and guaiacol. Furfural showed distribution coefficients of at least 1.27 in all three solvents. Guaiacol and TMO both showed a significantly lower lactic acid leaching than 2-MTHF. This makes them potential alternatives for 2-MTHF in this technique.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bio-based materials can be used in place of their fossil-based counterparts to lessen the environmental impact of consumer goods production, on the condition that the production method is efficient and uses as little CO2 and other harmful pollutants as possible. As a rich and concentrated source of non-fossil carbon, lignocellulosic biomass is regarded as a promising alternative to fossil resources for the production of sustainable fuels [1,2,3,4,5] and chemicals [6,7,8,9]. However, only a small portion of the photosynthetic capacity is consumed by human activities, resulting in significant resource waste [10]. Nowadays, kraft pul** method is used in the paper and pulp industry, one of the largest consumers of lignocellulose [11], to create energy and cost-efficient plants. Using this method, cellulose is extracted by lignocellulosic biomass delignification in a solution of sodium hydroxide and sodium sulfide. The isolated cellulose pulp is utilized in paper production [12]. Most of the remaining plant parts dissolved in the liquor are often burned to produce heat and to retrieve sodium sulfide [13,14,15]. Processes that can convert lignocellulose biomass into multiple products, such as cellulose, lignin, hemicellulose, or hemicellulose degradation products like furanics, are necessary to use this feedstock as efficiently as possible and to valorize materials rather than energy, thereby achieving sustainable production [1, 9].
In several processes, usually with organic solvents, such as organosolv [16] and OrganoCat [17, 18], burning of the lignin is avoided. One of these options uses deep eutectic solvents (DESs) as an alternative pul** method [19]. DESs consist of two or three components that form strong hydrogen bonds with one another in a eutectic mixture. The melting temperatures of DES mixtures drop significantly lower (> 50 °C) than expected for ideal mixtures [20]. Some of the DES components, such as halogen anions, can have catalytic behavior, making the DES-based pul** technique advantageous over conventional acid-based organosolv cooking [21, 22], while addition of water helps stabilize the DES against esterification, which does compromise DES stability in water-free environment at elevated temperatures [12]. The reactions that need to be catalyzed are the reactions that liberate lignin and hemicellulose from the wood matrix.
The first of these two, lignin, is a prominent DES-pul** byproduct, and an inhomogeneous organic polymer composed of three major aromatic monomers: guaiacyl, syringyl, and p-hydroxyphenyl [1, 8]. In addition, lignin contains different unit linkages responsible for important characteristic of lignin [23,24,25,26,27]. Because of the complexity of this polymer, separation, purification, and valorization of that are challenging [28,29,30,31]. Therefore, fractionation of heterogeneous lignin (molecular weight-dependent heterogeneity) into numerous homogeneous fractions is expected to be an effective method, easing the lignin valorization [32,33,34,35]. Next to the lignin, furanic compounds are one of the most important classes of intermediates that can be derived from the hemicellulose (and unfortunately also from some of the cellulose when it breaks down) in biomass and they can be further valorized to a wide variety of products with applications in bio-polymers, bio-diesel, pharmaceuticals, flavor/fragrance industry, and fine chemicals [2, 36,37,38]. Examples of hemicellulose-derived molecules are 5-hydroxymethyl-2-furfural (5-HMF) and furfural, arising from dehydration of hexose and pentose, respectively. 5-HMF and furfural may find applications in paints and varnishes, fuels, plastics, and composites [39,40,41], while furfural can act as solvent in many processes [42]. During pul** processes using acidic DES, hemicellulose degrades into hemicellulose fractions, which then react to form furanics. As a result, next to the fibers, a liquid DES outlet stream, so called DES-black liquor, includes among other constituents mainly lignin, hemicellulose, and furanics. The key to develop the DES-pul** process is the valorization of the various lignin fractions with varying molecular weights as well as the furanics from this stream [15, 25]. Therefore, it is essential to use technologies that are both economically viable and environmentally friendly in order to fractionate lignin and furanics from the stream in a sustainable way [43].
Several separation processes have been explored for extracting the lignin dissolved in the DES-black liquor, including membrane separation [43, 44], cold water precipitation, and liquid–liquid extraction (LLX) with an organic solvent [45, 46]. However, applying single separation technique would not be sufficient to completely regenerate DES and extract all fractions, including lignin [43, 46]. Smink et al. [45], found that after DES-delignification, lignin dissolved in lactic acid and choline chloride can be extracted into 2-methyltetrahydrofuran (2-MTHF). The separation process was hampered, however, by lactic acid leaching to the solvent phase [45]. Furthermore, they showed that cold water precipitation could fully recover lignin fractions with molecular weights larger than 5000 Da, while it could recover just 50% of the lignin fraction about 1000 Da. Therefore, combining water precipitation and LLX appears ideal for first isolating the larger lignin fraction, and then recover furanics and lower molecular weight lignin [46].
Since water precipitation of the higher molar weight lignin results in a diluted DES-based black liquor in water stream, the properties of this stream are significantly different from the DES-based black liquor coming directly from the cooker. As a result, more solvents may be applicable for this stream than was concluded in an earlier solvent selection study [45]. The immiscibility of lactic acid with solvent, or at least its restricted mutual miscibility, is of utmost importance for LLX. It is highly desirable to have high lignin and furanics distribution coefficients to allow for a low solvent to feed ratio. The selection of a solvent should also take into account its low water miscibility, so as to minimize water losses, as well as its low toxicity and low environmental impact.
From the earlier study [45], the solvents were reconsidered, and guaiacol was found immiscible with diluted DES-based black liquor and chosen for this study as an aromatic solvent that is naturally sourced and can be produced through catalytic depolymerization of lignin [47, 48]. Next to guaiacol that was selected from an earlier study, another alternative for 2-MTHF, is 2,2,5,5-tetramethyl oxolane (TMO). In contrary to the more well-known solvent, 2-MTHF, which is obtained from sugars, TMO is synthesized from acetylene and acetone [49]. This can be a bio-derived solvent if the acetylene is produced from biogas and acetone using the well-known acetone-butanol-ethanol (ABE) fermentation, due to its relatively low water miscibility, and the absence of the peroxide production risk that is associated with other ethers [49, 50]. TMO is a solvent of interest for potential future industrial applications. In addition to these solvents, m-xylene, toluene, and eugenol were added to the list for the solvent screening study based on their performance in the extraction of furanics from aqueous solutions as reported in the literature [
2 Materials and methods
2.1 Chemicals used
2-MTHF (> 99 wt.%, 250 ppm BHT), eugenol (> 98 wt.%), and guaiacol (natural, > 99 wt.%) were purchased from Sigma-Aldrich. TMO (99.5 wt.%), m-xylene (> 99 wt.%), and toluene (> 99.5 wt.%) were supplied by Addible, Riedel-de Haën and Acros Organics, respectively. Milli-Q water was used for sample preparation and analysis. DES-black liquor has been provided by Center technique du papier (CTP), where the biomass delignification had been done at 130 °C for 4 h, with DES to wood ratio of 8.6 [g/g] (100 kg oven-dried spruce chips from Sappi Austria Produktions-GmbH & Co. KG. was mixed with 860 kg DES consisting of lactic acid (88 wt%, rest water) and choline chloride (98%) in weight ratio of 10 to 1 [g/g]). Prior to the delignification process, the DES was prepared in a separate tank by mixing and pre-heating the DES to the reaction temperature. A Karl-Fischer titration showed a weight percentage of 21 ± 1% water in the DES-black liquor, being the combined water presence in the lactic acid prior to pul**, and water liberated from the wood during pul**.
2.2 Chemical analysis
2.2.1 GPC
Gel permeation chromatography (GPC) was used to determine the molar weight distribution of the samples. This was carried out using an Agilent 1260 Infinity series with a refractive index detector and a UV detector operating at 254 nm with a set of three GPC PLgel 3 m MIXED-E column series. The mobile phase of the column was tetrahydrofuran (THF) and water with a volume ratio of 95:5 (v:v) to allow the lignin to fully dissolve. Four milliliters of the mobile phase was added per 40 mg of sample. The operating condition of the GPC column was a flow rate of 1 mL/min at 40 °C. Utilizing polystyrene solutions with molecular weights ranging from 162 to 27,810 Da, molar weight distributions were calibrated. The results of UV absorbance were used to determine the molar weight distribution of samples. To calculate the mass ratio of the lignin in each sample, the product of UV absorbance corrected by dilution factor and density of each sample was used.
2.2.2 HPLC
High-performance liquid chromatography (HPLC) was used to quantify the components besides lignin in the samples. To accomplish this, an Agilent 1200 series was used with UV detector operating at 285 nm using a Hi-Plex-H column at 60 °C and a refractive index detector at 55 °C. The mobile phase was 5 mM sulphuric acid with flowrate of 0.6 mL/min. Samples with known concentrations have been applied to determine the response factors.
2.2.3 Karl-Fischer titration
Using a Metrohm 787 KF Titrino, a Karl-Fisher titration was performed to calculate the water content in the samples. A 20 mL burette of hydranal composite 5 (5 mg water/mL) was titrated in a 3:1 (v:v) solution of methanol and dichloromethane. Samples were measured in duplo with a relative error of less than 1%.
2.3 Experimental procedure
2.3.1 Cold water precipitation
Water and DES-black liquor were mixed at room temperature in various mass ratios of water to DES-black liquor from 1 to 5 to add up to a total of 15 g in a 15-mL Eppendorf centrifuge tube. The mixtures were shaken thoroughly and left overnight at room temperature. The solids were separated by centrifugation at 9000 rpm for 10 min. The precipitated lignin was collected on a petri dish and washed twice with 20 mL Milli-Q water. The sample was dried by leaving it in a vacuum oven at room temperature overnight. DES-black liquor, dried lignin, and the supernatant, so called aqueous DES-black liquor, were analyzed by GPC. All experiments were repeated three times. The precipitation yield of lignin was calculated using Eq. (1):
where \({w}_{\mathrm{lignin},\mathrm{aq}.\mathrm{DES}-\mathrm{BL}}\) and \({w}_{\mathrm{lignin},\mathrm{ DES}-\mathrm{BL}}\) represent, respectively, the mass fractions of lignin in the aqueous DES-black liquor following precipitation and the initial DES-black liquor in [g/g]. \({M}_{\mathrm{aq}.\mathrm{DES}-\mathrm{BL}}\) and \({M}_{\mathrm{DES}-\mathrm{BL}}\) are the mass of the aqueous DES-black liquor and the initial DES-black liquor in [g].
2.3.2 Liquid–liquid extraction (LLX)
For LLX experiments, the aqueous DES-black liquor after lignin precipitation was added to the solvent with solvent to feed mass ratio as 1:1. A Mettler AT200 analytical balance was used to weigh the components with an accuracy of 0.1 mg. The vials were shaken at 30 °C and 200 rpm using a Julabo F25 water bath to reach the equilibrium. Following extraction, the phases were settled for 1 h and then separated using needle and syringes. All experiments were repeated three times. HPLC was used to identify the components present and their composition in the raffinate and solvent phases. Mass balance was employed to determine the concentration of components in solvent phases when HPLC analysis was not possible due to low solubility in the HPLC eluent. The lignin apparent molar weight distribution was found using GPC. Karl-Fischer was used to determine the water content before and after LLX.
The distribution coefficient of each component, \({D}_{i},\) were measured according to Eq. (2):
where \({w}_{i,\mathrm{Solvent}}\) and \({w}_{i, \mathrm{Raffinate}}\) are the measured mass fraction of component \(i\) in the solvent and the raffinate phase after LLX in [g/g], respectively.
The total lignin extraction yield in proposed process was calculated by Eq. (3):
where \({w}_{\mathrm{lignin},\mathrm{ Raffinate}}\) is the measured mass fraction of lignin in the raffinate phase in [g/g], and \({M}_{\mathrm{raffinate}}\) is the mass of the raffinate phase in [g] after LLX.
3 Results and discussion
3.1 Cold water precipitation
Water precipitation with different amounts of water was performed to investigate recovery of different molar mass fractions of lignin using water as anti-solvent. The mass ratios of lignin in DES-black liquor and in the aqueous DES-black liquor after separating precipitated lignin were measured using GPC, and the yield was calculated using Eq. (1) with the standard deviation of 10–15%. Figure 1 shows lignin precipitation yield as function of apparent lignin molar mass. Even though the figure illustrates that adding additional water increases lignin precipitation yield, by adding water with ratio of 3.5:1 [g/g] and more to DES-black liquor, the recovery of lignin remains constant. However, for smaller lignin fractions (< 5000 Da), which are partially soluble in water [46], the average yield is around 70% for larger amounts of water and will drop to almost 50% for a water to DES-black liquor ratio of 1:1 [g/g]. The precipitation yield for lignin fractions larger than 5000 Da is always greater than 80%. This confirms that the lignin’s hydrophobicity increases with its molecular weight [57, 58].
3.2 Liquid–liquid extraction (LLX)
3.2.1 Solvent screening
In the regeneration process of DES after delignification using LLX, the ideal solvent must have high distribution coefficients for lignin and furanics and low distribution coefficients for lactic acid, resulting in less lactic acid leaching to the solvent. Therefore, to find the suitable solvent, after precipitation using a water to DES-black liquor ratio of 1:1 [g/g], the aqueous DES-black liquor was equilibrated with six different solvents (Table 1) at a solvent to feed ratio of 1:1 [g/g] (solvent/aqueous DES-black liquor). Following phase formation, the solvent and raffinate phases were separated and analyzed using HPLC, GPC, and Karl Fischer, as was described in Sect. 2.3.2. The suitability of these solvents was discussed based on the distribution coefficients of furfural, 5-HMF, lactic acid, and smaller lignin fractions (< 5000 Da) that were calculated using Eq. (2).
Figure 2(a) shows the distribution coefficients for lignin fractions smaller than 5000 Da for different solvents. From this figure, no lignin was removed by toluene or m-xylene from the precipitated aqueous DES-BL. Moreover, the largest lignin distribution coefficients were found in 2-MTHF and guaiacol. The hydrophilic nature of the lignin that remained in the DES-black liquid after the precipitation step suggests that the polarity of the solvents can explain these results [45]. Hence, it is challenging to extract the lower molecular weight lignin using more apolar solvents. TMO and eugenol were found to be able to remove lignin as well.
The distribution coefficients of smaller fracions of lignin (< 5000 Da) (a) and furfural, 5-HMF and lactic acid (b) between aqoueus DES-black liquor (1:1 [g/g] water to DES-black liquor mass ratio in the precipitation step) and different solvents used in this work. All LLX experiments were done at 30 °C
All six solvents demonstrated a furfural distribution coefficient greater than 2, as seen in Fig. 2(b). However, when comparing the distribution coefficients of lactic acid and 5-HMF, 2-MTHF, guaiacol, TMO, and eugenol showed the most encouraging results and were therefore chosen as the preferred solvents for further investigation. Given that eugenol’s molecular structure contains reactive functional groups, including allyl, hydroxyl, and methoxy [59] and in the acidic environment of DES-black liquor may react with furfural [60], it is challenging to use this solvent. Therefore, 2-MTHF, guaiacol, and TMO were chosen to explore the LLX process in wider context with various amounts of water added to the DES-black liquor during the precipitation step.
3.2.2 Extensive LLX study
To get more insight in the LLX step in DES regeneration process using selected solvents, LLX experiments with 2-MTHF, guaiacol, and TMO have been executed with the feed of various amounts of water added to the DES-black liquor during the precipitation step and a solvent to feed ratio of 1:1 [g/g]. LLX experiments using TMO as the solvent resulted in crud formation for feeds of water to DES-black liquor that were less than 2:1 [g/g]. This was most likely caused by the presence of higher molecular weight lignin with hydrophobicity characteristic in the aqueous DES-black liquor and lower pH due to the higher lactic acid concentration [30]. However, by adding more water to DES-black liquor in precipitation stage which removes more higher molecular weight lignin, liquid extraction resulted in the formation of transparent and homogenous phases.
Furanics extraction
5-HMF and furfural distribution coefficients in LLX experiments using guaiacol, 2-MTHF, and TMO have been calculated using Eq. (2). The results are presented in Fig. 3(a) and (b). According to these figures, the distributions of furfural and 5-HMF in guaiacol are greater than those of 2-MTHF and TMO. Increasing the amount of water added to DES-black liquor during the precipitation process causes a slight increase in the furfural distribution coefficients. Similarly to furfural, increasing the water content of DES-black liquor during the precipitation process decreases 5-HMF’s affinity for the aqueous DES-black liquor, resulting in a greater distribution coefficient. Furthermore, the distribution coefficient of furfural is greater than that of 5-HMF in all solvents. However, it appears that the distribution coefficients for 5-HMF in the TMO system are not a function of the amount of water in DES-black liquor and are always around 0.4. Since guaiacol is miscible with the DES-black liquor in the absence of water, extraction results were only reported for 2-MTHF and TMO in LLX experiments where no water was introduced to the DES-black liquor.
Leaching of lactic acid and water to solvent
Regeneration of DES after delignification with minimum DES component loss was one of the primary objectives of the study on the recovery techniques in this study. Therefore, next to the lignin and furanics extraction, understanding the leaching of lactic acid into the solvent phase is a key aspect, and of importance to compare the performances of all solvents with each other. The greater the distribution coefficients of lactic acid and water, the higher their leaching into the solvent. Figure 4(a) shows for varied water to DES-black liquor ratios in the distribution coefficients of lactic acid in the 2-MTHF system are always close to 1, resulting in massive lactic acid losses during LLX. For the other two solvents TMO and guaiacol in contrary, the lactic acid distribution coefficients are significantly lower than in 2-MTHF.
As shown in Fig. 4(b), the distribution coefficient of water into solvents is decreasing for all three systems as the amount of water in the feed is increased. TMO’s miscibility in water is significantly lower than that of guaiacol and 2-MTHF, owing to its less polar structure as a result of its four methyl groups [49].
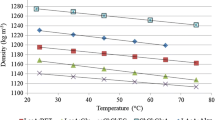
The equilibrium mass fractions of water and lactic acid concentration in the solvent phase has been shown in Fig. 5. For all three solvents, decreasing the lactic acid content in the feed by increasing the amount of water added to DES-black liquor in the precipitation step reduces the lactic acid leaching to the solvent. With increasing lactic acid concentration in the solvent phase, the solvent phase water content increases. More lactic acid leaching into the solvent phase enhances the hydrophilicity of the solvent, hence increasing water leaching [61, 62]. In the case of LLX without lignin precipitation for 2-MTHF and TMO, the solvent phases contain the maximum concentration of lactic acid since the feed contains less water thus providing less hydrophilic attraction to prevent the lactic acid leaching.
Water soluble lignin extraction
Equation (2) was used to express the distribution coefficients of lignin over the solvent and raffinate phases. Using the GPC spectra (VWD), it was possible to determine the mass fractions of lignin. Figure 6(a) to (c) display the lignin distribution coefficients (Mw < 5000 Da) at varying solvent to water-diluted DES ratios for all three solvents. Displayed results are averages of multiple repeats with the standard deviation of 10–15%; the standard deviation becoming larger as the remaining concentration of larger lignin fractions (> 5000 Da) in the aqueous DES-black liquor after precipitation becomes lower, and ultimately, the distribution coefficient for these fractions could not be measured. An example of GPC spectrum for DES-black liquor, aqueous DES-black liquor, raffinate, and solvent phases following LLX with three different solvents is given in the supplementary information. As can be seen in Fig. 6, the three examined solvents exhibit distinct behavioral differences. Whereas the distribution coefficient of water-soluble lignin (Mw < 5000 Da) over aqueous DES-black liquor and 2-MTHF phases is hardly dependent on the ratio of added water to DES-black liquor in the precipitation step, guaiacol exhibits a maximum lignin distribution at an intermediate water to DES-black liquor ratio, and TMO exhibits an increasing lignin distribution at an increasing water to DES-black liquor ratio, but the absolute values are much lower than for the other two solvents.
Lignin distribution coefficient as function of apparent lignin molar mass for 2-MTHF (a), guaiacol (b), TMO (c), and for various water to DES-black liquor mass ratios in the precipitation step: 5:1 (solid, blue), 3.5:1 (dot, red), 2:1 (dash, black), and 1:1 (dash-dot, green). All LLX experiments were done at 30 °C
All three solvents have an ether functionality, and guaiacol additionally has a phenolic OH-group in addition to the ether. Considering the fact that smaller lignin molecules are aromatic and oxygenic in nature, hydrogen-bonding interactions with the solvents are anticipated [63, 64], and the hydrophobic property “like dissolves like” will also be important [65, 66]. Guaiacol is the only solvent that is a hydrogen bond donor. This is anticipated to have a considerable effect on the interaction energy [67] between the solvent and lignin. Guaiacol has high distribution coefficients for the smaller fractions of lignin (i.e., lignin < 2500 Da) as well as the lignin fractions with higher hydrophobicity (i.e., lignin in the range of 2500–5000 Da). This can be explained by the hydrogen-bond capacity of guaiacol, which might reduce the intermolecular hydrogen bond interactions with lignin and break them down to the smaller fractions while mixing with the solvent and making it easier for their extraction into the solvent [68, 69]. As a result, the distribution coefficients for the lignin fractions in the range of 2500–5000 Da are infinite and independent of the feed’s water content.
The smaller fractions of lignin, particularly those less than 2500 Da, can be extracted using 2-MTHF and TMO, although their affinity for the larger and more hydrophobic lignin reduces when water to DES-black liquor ratio is less than 2:1 [g/g]. When comparing the lignin distribution coefficient for TMO and 2-MTHF systems, 2-MTHF shows higher values. Considering the structure of these two solvents, the main distinction is that the oxygen atom in TMO is significantly more crowded in TMO because of the four methyl groups around it. The difference between TMO and 2-MTHF in extraction of lignin can also be explained by the solvent’s water content. The presence of water in solvent will increase the concentration of hydrogen bonds between lignin and solvent, allowing higher solubility of lignin in solvent [70, 71]. The water content of the solvent phase after extraction has been discussed in the previous section. Since water content in 2-MTHF is higher than TMO, enhanced lignin extraction is expected. Crud formation for feed ratios of less than 2:1 [g/g] of water to DES-black liquor can also be a factor for limiting the TMO for extraction of larger molecular weights (i.e., lignin in range of 2500–5000 Da). It is important to note that the concentration of lignin larger than 2500 Da is low for feeds with added water to DES-black liquor with a ratio higher than 2:1 [g/g], likely enough to reach its solubility limit in solvents. As a result, no lignin fractions in this range of molecular weight were found in the raffinate phases after extraction with all three solvents, leading to an infinite distribution coefficient.
These extraction systems are complex, as evidenced by the fact that only a few solvents were initially found to be suitable during the previous study [45], due to the limited window for the two-phase formation with the DES-black liquor without adding water. Therefore, it is interesting that these solvents exhibit such distinctly different behavior for diluted DES-black liquor.
Equation (3) was used to calculate the total yield for lignin extraction in proposed process using varius solvents. Figure 7(a) to (c) show the total extraction yield of lignin in the process of precipitation and a LLX using 2-MTHF and guaiacol and TMO, respectively. From these figures, it appears that for water to DES ratios of 3.5:1 [g/g] and higher, LLX can be used to recover nearly all of the lignin that remains in the aqueous DES-black liquid after precipitation. It appears that multiple stages LLX will allow for the extraction of all lignin with less water. Additionally, 2-MTHF and guaiacol extract water-soluble lignin very effectively; however, guaiacol has a greater extraction yield for feeds containing less water, especially for larger lignin fractions, resulting in fewer stages being needed to properly recover the lignin and requiring less solvent overall. In account of the lower distribution coefficient of smaller lignin for TMO (Fig. 6), it is expected that the total extraction yield will not differ considerably from the precipitation yield when a single liquid extraction stage is performed.
Total extraction yield for lignin as function of apparent lignin molar mass for 2-MTHF (a), guaiacol (b), TMO (c), and for various water to DES-black liquor (DES-BL) mass ratios in the precipitation step: 5 (solid, blue), 3.5 (dot, red), 2 (dash, black), and 1 (dash-dot, green). All LLX experiments were done at 30 °C
4 Conclusion
To extract lignin and furanics (such as furfural and 5-HMF) from a DES comprised of choline chloride and lactic acid, water precipitation followed by LLX was applied. A solvent comparison study with six different solvents was performed for LLX. 2-MTHF, guaiacol, and TMO were found as suitable solvents for recovery of lignin and furanics. By varying the amount of water in the precipitation step, it showed that with increasing water addition to DES-black liquor, the precipitation yield of lignin increases until a water:DES black liquor ratio of 3.5:1. For the subsequent LLX, all three successful solvents extract with a total extraction yield higher than 45% for smaller lignin (500–5000 Da) and higher than 70% for lignin (> 5000 Da) when the water to DES-black liquor in the precipitation is at least 1:1 [g/g]. 2-MTHF and guaiacol demonstrated comparatively higher distribution coefficients for smaller lignin fractions (< 5000 Da) than TMO. Whereas the lower lignin extraction in TMO relative to 2-MTHF is logically explained by the difference in steric hindrance and solvent’s water content, the trend in the lignin extraction for guaiacol is more complicated.
All three solvents extracted furfural with distribution coefficients higher than 1, with the higher values for guaiacol. Although the distribution coefficient of 5-HMF in TMO system is around 0.4, which is not too high, on the other hand, lactic acid and water distribution coefficients are also low for TMO. Also for guaiacol, leaching of water and lactic acid is lower than for 2-MTHF, facilitating the recovery of furanics and lignin from solvent phases without fractionation of DES components. For all three solvents, adding more water to DES-black liquor in precipitation step seems to improve the recovery of furanics and smaller lignin in the LLX step and reduces the leaching of lactic acid and water.