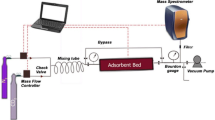
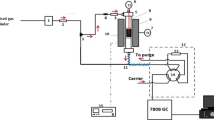
Abstract
Mitigation of carbon dioxide emitted from burning fossil fuels is essential to overcome climate change issues. Adsorption technology could significantly help in capturing CO2 and, thereby minimizing global warming with low-cost penalties. Mg-MOF-74 was reported as a distinguished adsorbent that has high adsorption capacity at flue gas conditions. In the present study, an improvement of crystallinity, porosity, and capacity of Mg-MOF-74 was investigated through controlling the heat surface area of the sample solution during the synthesis process. The results showed that the increase in the heat surface area during the synthesis process increased BET surface area and pore volume of the adsorbent by 38% and 44%, respectively, over those obtained by the reported method in the literature. For additional improvement in the cyclic CO2 uptake, multi-walled carbon nanotubes (MWCNT) were incorporated with Mg-MOF-74. The adsorption cycling stability was performed using three techniques: temperature swing adsorption (TSA), vapor swing adsorption (VSA), and temperature vacuum swing adsorption (TVSA). It was observed that the incorporation of MWCNT with Mg-MOF-74 resulted in higher CO2 recycling capacity (14.4%) using thermal-based regeneration processes (i.e., TSA and TVSA) due to the enhancement in the thermal transport properties of the new composite (MWCNT/Mg-MOF-74).








Similar content being viewed by others
Abbreviations
- BET:
-
Brunauer–Emmett–Teller
- CAS:
-
Chemical abstracts service
- CCS:
-
Carbon capture and consequence
- CNT:
-
Carbon nanotubes
- ESA:
-
Electric swing adsorption
- GHG:
-
Greenhouse gases
- LAHT:
-
Large area for heat transfer
- MAHT:
-
Medium area for heat transfer
- MWCNT:
-
Multi-walled carbon nanotube
- MOF:
-
Metal–organic framework
- SAHT:
-
Small area for heat transfer
- TSA:
-
Temperature swing adsorption
- TVSA:
-
Temperature vacuum swing adsorption
- VSA:
-
Vacuum swing adsorption
- ZIF:
-
Zeolitic imidazolate framework
References
Ho, T.M.; Howes, T.; Bhandari, B.R.: Encapsulation of gases in powder solid matrices and their applications: a review. Powder Technol. 259, 87–108 (2014). https://doi.org/10.1016/j.powtec.2014.03.054
Cleugh, H.: Climate Change: Science and Solutions for Australia. CSIRO Publishing, Collingwood (2011)
Taub, D.R.; Miller, B.; Allen, H.: Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Glob. Change Biol. 14, 565–575 (2008). https://doi.org/10.1111/j.1365-2486.2007.01511.x
Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W.: Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science (80-) 326, 123–125 (2009). https://doi.org/10.1126/science.1176985
D’Alessandro, D.M.; McDonald, T.: Toward carbon dioxide capture using nanoporous materials. Pure Appl. Chem. (2010). https://doi.org/10.1351/pac-con-10-09-18
Advancing the Science of Climate Change. Washington, DC: National Academies Press (2010) https://doi.org/10.17226/12782
IPCC. Climate Change 2007: Synthesis Report (2007). https://doi.org/10.1256/004316502320517344
The Carbon Capture and Storage Association (CCSA). http://www.ccsassociation.org
Choi, S.; Drese, J.H.; Jones, C.W.: Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2, 796–854 (2009). https://doi.org/10.1002/cssc.200900036
Gibson, J.A.A.; Mangano, E.; Shiko, E.; Greenaway, A.G.; Gromov, A.V.; Lozinska, M.M.; et al.: Adsorption materials and processes for carbon capture from gas-fired power plants: AMPGas. Ind. Eng. Chem. Res. 55, 3840–3851 (2016). https://doi.org/10.1021/acs.iecr.5b05015
Abanades, J.C.; Arias, B.; Lyngfelt, A.; Mattisson, T.; Wiley, D.E.; Li, H.; et al.: Emerging CO2 capture systems. Int. J. Greenh. Gas Control 40, 126–166 (2015). https://doi.org/10.1016/j.ijggc.2015.04.018
Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; et al.: Carbon capture by physical adsorption: materials, experimental investigations and numerical modeling and simulations—a review. Appl. Energy (2016). https://doi.org/10.1016/j.apenergy.2015.10.011
Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R.: Post-combustion CO2 capture using solid sorbents: a review. Ind. Eng. Chem. Res. 51, 1438–1463 (2012). https://doi.org/10.1021/ie200686q
Chomiak, K.; Gryglewicz, S.; Kierzek, K.; Machnikowski, J.: Optimizing the properties of granular walnut-shell based KOH activated carbons for carbon dioxide adsorption. J. CO2 Util. 21, 436–443 (2017). https://doi.org/10.1016/j.jcou.2017.07.026
Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F.: Comparative study of CO2 capture by carbon nanotubes, activated carbons, and zeolites. Energy Fuels 22, 3050–3056 (2008). https://doi.org/10.1021/ef8000086
Rashidi, N.A.; Yusup, S.: Potential of palm kernel shell as activated carbon precursors through single stage activation technique for carbon dioxide adsorption. J. Clean. Prod. 168, 474–486 (2017). https://doi.org/10.1016/j.jclepro.2017.09.045
Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P.; Poston, J.A.: Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 15, 279–284 (2001). https://doi.org/10.1021/ef000241s
Qasem, N.A.A.; Ben-Mansour, R.; Habib, M.A.: Enhancement of adsorption carbon capture capacity of 13X with optimal incorporation of carbon nanotubes. Int. J. Energy Environ. Eng. 8, 219–230 (2017). https://doi.org/10.1007/s40095-017-0235-7
Gholipour, F.; Mofarahi, M.: Adsorption equilibrium of methane and carbon dioxide on zeolite 13X: experimental and thermodynamic modeling. J. Supercrit. Fluids 111, 47–54 (2016). https://doi.org/10.1016/j.supflu.2016.01.008
Silva, J.A.C.; Cunha, A.F.; Schumann, K.; Rodrigues, A.E.: Binary adsorption of CO2/CH4 in binderless beads of 13X zeolite. Microporous Mesoporous Mater. 187, 100–107 (2014). https://doi.org/10.1016/j.micromeso.2013.12.017
Mcewen, J.; Hayman, J.; Yazaydin, A.O.: A comparative study of CO2, CH4 and N2 adsorption in ZIF-8, Zeolite-13X and BPL activated carbon. Chem. Phys. 412, 72–76 (2013). https://doi.org/10.1016/j.chemphys.2012.12.012
Abdelnaby, M.M.; Qasem, N.A.A.; Al-Maythalony, B.A.; Cordova, K.E.; Al Hamouz, O.C.S.: A microporous organic copolymer for selective CO2 capture under humid conditions. ACS Sustain. Chem. Eng. (2019). https://doi.org/10.1021/acssuschemeng.9b02334
Abdelnaby, M.M.; Alloush, A.M.; Qasem, N.A.A.; Al-Maythalony, B.A.; Mansour, R.B.; Cordova, K.E.; et al.: Carbon dioxide capture in the presence of water by an amine-based crosslinked porous polymer. J. Mater. Chem. A (2018). https://doi.org/10.1039/c8ta00012c
Alloush, A.M.; Abdelnaby, M.M.; Cordova, K.E.; Qasem, N.A.A.; Al-Maythalony, B.A.; Jalilov, A.; et al.: Selectively capturing carbon dioxide from mixed gas streams using a new microporous organic copolymer. Microporous Mesoporous Mater. (2020). https://doi.org/10.1016/j.micromeso.2020.110391
Wen, Z.; Chen, W.; Li, Y.; Xu, J.: A theoretical mechanism study on the ethylenediamine grafting on graphene oxides for CO2 capture. Arab. J. Sci. Eng. 43, 5949–5955 (2018). https://doi.org/10.1007/s13369-018-3087-4
Qasem, N.A.A.; Qadir, N.U.; Ben-Mansour, R.; Said, S.A.M.: Synthesis, characterization, and CO2 breakthrough adsorption of a novel MWCNT/MIL-101(Cr) composite. J. CO2 Util. 22, 238–249 (2017). https://doi.org/10.1016/j.jcou.2017.10.015
Sarfraz, M.; Ba-Shammakh, M.: Combined effect of CNTs with ZIF-302 into polysulfone to fabricate MMMs for enhanced CO2 separation from flue gases. Arab. J. Sci. Eng. 41, 2573–2582 (2016). https://doi.org/10.1007/s13369-016-2096-4
Pentyala, V.; Davydovskaya, P.; Ade, M.; Pohle, R.; Urban, G.: Carbon dioxide gas detection by open metal site metal organic frameworks and surface functionalized metal organic frameworks. Sens. Actuators B Chem. 225, 363–368 (2016). https://doi.org/10.1016/j.snb.2015.11.071
Campbell, J.; Tokay, B.: Controlling the size and shape of Mg-MOF-74 crystals to optimise film synthesis on alumina substrates. Microporous Mesoporous Mater. 251, 190–199 (2017). https://doi.org/10.1016/j.micromeso.2017.05.058
Qasem, N.A.A.; Ben-Mansour, R.: Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74. Appl. Energy 230, 1093–1107 (2018). https://doi.org/10.1016/j.apenergy.2018.09.069
Ben-Mansour, R.; Qasem, N.A.A.; Antar, M.A.: Carbon dioxide adsorption separation from dry and humid CO2/N2 mixture. Comput. Chem. Eng. (2018). https://doi.org/10.1016/j.compchemeng.2018.06.016
Ben-Mansour, R.; Abuelyamen, A.; Qasem, N.A.A.: Thermal design and management towards high capacity CO2 adsorption systems. Energy Convers. Manag. 212, 112796 (2020). https://doi.org/10.1016/j.enconman.2020.112796
Adhikari, A.K.; Lin, K.-S.: Improving CO2 adsorption capacities and CO2/N2 separation efficiencies of MOF-74(Ni,Co) by do** palladium-containing activated carbon. Chem. Eng. J. 284, 1348–1360 (2016). https://doi.org/10.1016/j.cej.2015.09.086
Lee, D.J.; Li, Q.; Kim, H.; Lee, K.: Preparation of Ni-MOF-74 membrane for CO2 separation by layer-by-layer seeding technique. Microporous Mesoporous Mater. 163, 169–177 (2012). https://doi.org/10.1016/j.micromeso.2012.07.008
Cho, H.Y.; Yang, D.A.; Kim, J.; Jeong, S.Y.; Ahn, W.S.: CO2 adsorption and catalytic application of Co-MOF-74 synthesized by microwave heating. Catal. Today 185, 35–40 (2012). https://doi.org/10.1016/j.cattod.2011.08.019
Ben-Mansour, R.; Qasem, N.A.A.; Habib, M.A.: Adsorption characterization and CO2 breakthrough of MWCNT/Mg-MOF-74 and MWCNT/MIL-100(Fe) composites. Int. J. Energy Environ. Eng. (2018). https://doi.org/10.1007/s40095-018-0260-1
Ben-Mansour, R.; Qasem, N.A.A.: An efficient temperature swing adsorption (TSA) process for separating CO2 from CO2/N2 mixture using Mg-MOF-74. Energy Convers. Manag. 156, 10–24 (2018). https://doi.org/10.1016/j.enconman.2017.11.010
Qasem, N.A.A.; Ben-Mansour, R.: Energy and productivity efficient vacuum pressure swing adsorption process to separate CO2 from CO2/N2 mixture using Mg-MOF-74: a CFD simulation. Appl. Energy 209, 190–202 (2018). https://doi.org/10.1016/j.apenergy.2017.10.098
Wang, L.J.; Deng, H.; Furukawa, H.; Gándara, F.; Cordova, K.E.; Peri, D.; et al.: Synthesis and characterization of metal-organic framework-74 containing 2, 4, 6, 8, and 10 different metals. Inorg. Chem. 53, 5881–5883 (2014). https://doi.org/10.1021/ic500434a
Yang, D.-A.; Cho, H.-Y.; Kim, J.; Yang, S.-T.; Ahn, W.-S.: CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method. Energy Environ. Sci. 5, 6465–6473 (2012). https://doi.org/10.1039/c1ee02234b
Acknowledgements
The support received from Deanship of Research (DSR), KFUPM, under Project SB191021, is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qasem, N.A.A., Abuelyamen, A. & Ben-Mansour, R. Enhancing CO2 Adsorption Capacity and Cycling Stability of Mg-MOF-74. Arab J Sci Eng 46, 6219–6228 (2021). https://doi.org/10.1007/s13369-020-04946-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04946-0