Abstract
Recently, nanoparticle-based drug delivery systems have been widely used for the treatment, prevention, and detection of diseases. Improving the targeted delivery ability of nanoparticles has emerged as a critical issue that must be addressed as soon as possible. The bionic cell membrane coating technology has become a novel concept for the design of nanoparticles. The diverse biological roles of cell membrane surface proteins endow nanoparticles with several functions, such as immune escape, long circulation time, and targeted delivery; therefore, these proteins are being extensively studied in the fields of drug delivery, detoxification, and cancer treatment. Furthermore, hybrid cell membrane-coated nanoparticles enhance the beneficial effects of monotypic cell membranes, resulting in multifunctional and efficient delivery carriers. This review focuses on the synthesis, development, and application of the cell membrane coating technology and discusses the function and mechanism of monotypic/hybrid cell membrane-modified nanoparticles in detail. Moreover, it summarizes the applications of cell membranes from different sources and discusses the challenges that may be faced during the clinical application of bionic carriers, including their production, mechanism, and quality control. We hope this review will attract more scholars toward bionic cell membrane carriers and provide certain ideas and directions for solving the existing problems.
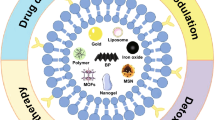
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is defined as a branch of science, engineering, and technology that involves molecules at the nanoscale (1–100 nm). To date, nanotechnology has contributed to several scientific fields, such as chemistry, physics, biology, and medicine. In particular, in biomedicine [1], many novel and promising nanoparticle (NP)-based drug delivery systems (DDSs) have been used for the safe and efficient transport of drugs or therapeutic genes in vivo [2]. Controlled distribution and drug release of NPs due to their nanoscale properties could improve bioavailability in vivo [2]. For example, because of their enhanced permeability and retention (EPR) effect [3,4,5,6], NPs can highly accumulate in tumor tissues. Even if NP delivery systems can achieve passive targeting, problems such as interaction with the reticuloendothelial system (RES), formation of a protein crown, accelerated blood clearance (ABC), and poor targeting ability toward specific cells remain unresolved [7,8,9,10,11]. Polyethylene glycol (PEG), designated as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA), is widely used for the surface modification of NPs in order to extend their blood circulation time and enhance their targeting capabilities [12]. The PEG chains form a flexible polymer brush layer and create steric hindrance, which can cover up the NP surface charge [13, 14]. This significantly inhibits the adsorption of serum proteins, thereby reducing the recognition of macrophages and minimizing complement activation [15]. However, recent clinical research has indicated the existence of anti-PEG immunity, suggesting that PEGylation can also lead to the ABC phenomenon [16,17,49]. In industrial-scale production, homogenization combined with centrifugation is extensively used [37, 38, 49].
Principal types of NP templates
Different inner cores endow CMC-NPs with different properties. There are two main types of inner cores: organic and inorganic. Core selection according to the subsequent application is necessary.
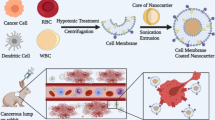
Organic inner cores have better biocompatibility and biodegradability [50, 51]. The US FDA has approved the clinical application of gelatin, liposome, and poly(lactic-co-glycolic acid) (PLGA). Among all inner cores, PLGA is the most commonly used in the preparation of membrane biomimetic carriers and holds great promise for clinical applications [52]. Various membranes, including platelet membranes [21, 53], cancer cell membranes [22, 54], macrophage membranes [55], and stem cell membranes [56], can be modified on PLGA particles to prevent the formation of agglomerates on NPs and achieve better delivery efficiency. Another widely used inorganic inner core is a liposome, which resembles the cell membrane [57, 58]. Liposomes are biodegradable colloids capable of containing hydrophobic or hydrophilic pharmaceuticals [59, 60]. Moreover, they can penetrate in vivo barriers as they are flexible [60]. Cell membrane coating improves the stability of phospholipid membranes and achieves a longer circulation time without affecting the drug loading capacity [61, 62].
The stability of inorganic NPs and their resistance to enzymatic degradation are unmatched [63]. Moreover, by manipulating the form, size, composition, and surface qualities of inorganic NPs, their inherent electrical, optical, and magnetic capabilities can be enhanced to achieve full therapeutic potential [63]. For example, an innovative class of nanophotothermal transduction agents, Fe3O4 NPs, can be designed for use in photothermal therapy (PTT) [28]. Macrophage membrane-coated Fe3O4 NPs can specifically target cancer cells and selectively kill cells by increasing the ambient temperature when exposed to laser light [64]. Another example is the use of stem cell membrane-camouflaged superparamagnetic iron oxide (SPIO) NPs for thermomagnetic therapy. SPIO NPs can rapidly change their magnetic moments and thus generate heat under a high-frequency alternating magnetic field for hyperthermia therapy applications [65]. When using inorganic nanocarriers, toxicity and biodistribution continue to be key concerns. Changing the particle size is one solution [66]. For instance, micron-sized CuO could result in safe delivery; however, CuO NPs could cause DNA damage [66,67,68]. In the case of SiO2, an increase in particle size (from 30–40 to 100–150 nm) could significantly reduce cytotoxicity [8) [28].
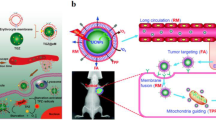
DiRL labeled liposomal nanoparticles (DiRL, LM-DiRL, TM-DiRL, and LTM-DiRL) (n = 4). a In vivo biodistribution of different groups after intravenous injection. b Quantitative analysis of fluorescence accumulation in the main organs. c Histogram of quantitative analysis of fluorescence accumulation in the main organs. Reproduced with permission [28]. DiRL, DiR-labeled liposomal nanoparticles; LM-DiRL, leukocyte membrane-coated DiRL; TM-DiRL, tumor cell membrane-coated DiRL; LTM-DiRL, leukocyte-tumor cell membrane-coated DiRL.
LCMs can also be used for cancer detection, playing an important role in cancer monitoring and diagnosis. Some existing detection methods are not sensitive and accurate enough for capturing and detecting circulating tumor cells (CTCs). They fail to predict tumor metastasis in advance because of the low concentration of CTCs and interference from leukocytes [178]. However, LCMs can reduce interference from homologous leukocytes and have the ability of tumor region targeting, which can improve CTC isolation and detection. For instance, Ding et al. successfully built a nanoplatform with LCMs for highly efficient cancer detection [171]. The purity of captured CTCs in the LCM-coated NPs group was 96.96%, which was much higher than that in the bare NPs and monotypic cell membrane-coated NPs groups.
In conclusion, LCMs can be extensively used for disease treatment, particularly in cancer therapy. Leukocytes have also been confirmed to be a precursor of tumor metastasis in human bodies. Therefore, some studies have focused on the regulation of epigenetic expression of the parent cell by LCMs and expression of a specific antigen profile for performing immunotherapy in order to enable efficient removal of tumor cells and cancer treatment [89].
This section reviews the characteristics and advantages of various types of HCMNs. More applications and experiments of HCMNs are presented in Table 2 for better understanding. In summary, several reports have indicated that different cell membrane combinations play unique roles in the treatment of specific diseases. HCMNs can have multiple applications, use in liquid biopsy and cancer vaccines, targeting disease regions, use in combination with other treatments, and detoxification.
Prospects and challenges
Cell membrane coating utilizes natural components at the source to directly transfer natural properties displayed by source cells, thereby recreating complex biological functions and integrating functions that cannot be achieved through synthesis. In this review, the drug delivery capabilities of CMC-NPs are highlighted. Biologically derived raw materials offer a longer blood circulation time, better immune escape, and stronger targeting ability than bare NPs. Undeniably, CMC-NPs still have drawbacks and pose obstacles. Their prospects and challenges will be the main topics of this section.
Quality control
As CMC-NP is a novel drug delivery platform, its quality control needs to be further explored. By referring to the existing standards and quality control specifications for cellular medicines [179, 180], the quality control of CMC-NPs can be divided into three parts.
Cell collection and isolation process control
In the case of cellular raw materials used for preparing CMC-NPs, cell identification, survival and growth activity assessment, foreign pathogen detection, and basic cell characteristic assessment are necessary. Cell characteristics include specific populations of cell surface markers, expression products, and differentiation potentials.
In addition, standard operation and management procedures for the collection and separation of different cells should be formulated and strictly implemented based on GMP requirements. Moreover, each cell type requires standardized and well-established cell culture protocols so that its phenotype and purity can be maintained during passaging [181].
Manufacturing process and storage ability
More consideration needs to be given to the fusion process. Careful calculation and control of the membrane-to-NP ratio are essential to ensure complete coverage and reduce loss of cell membrane. Moreover, the preparation of HCMNs is complex (e.g., determination of the ratio of the two cell membranes and the membrane mixing type), making it difficult to determine an optimal HCMN preparation method suitable for a particular disease [25]. Furthermore, producers are required to use standard biotechnological production and purification techniques. The entire production process should not lead to further impurities other than those originating from the active substance.
Sterilization is another important part of manufacturing process control. The currently accepted sterility assurance level (SAL) is 10−6 [182]. Quality control systems need to guarantee that pyrogens, bacteria, virus endotoxins, or LPS do not contaminate CMC-NPs. Filter sterilization is a widely used technique for sterilizing nanoformulations [183, 184]. Specific standards for sterility and endotoxin testing can be formulated according to national quality control regulations.
During the storage process, biological sample storage is usually performed using the freeze–drying method [185]. The potential influence of the lyophilization process on finished product quality results in product-derived impurities, which need to be controlled using the established analytical methods. In addition, the purity and coverage of the preparation process can impact the storage stability of different cell membrane coating systems [24, 31]. Therefore, numerous pre-experiments on screening conditions in the early stage of mass production are required to improve the storage stability of certain CMC-NPs.
Product control, batch analysis, and product stability
For analyzing the active substance quality in CMC-NPs, therapeutic activity, encapsulation rate, and drug release rate are assessed. The precise ingredients in each CMC-NP primarily vary in two areas: safety and efficacy.
To ensure batch-to-batch repeatability during mass production, process parameters must be examined to determine the variables that could harm the product. Process variables include ambient conditions (temperature, pH, and pressure), formulation variables (cell types, component ratios, and solvents utilized), and formulation processes (time, speed, flow conditions, and power) [186]. Short-, medium-, and long-term stability must also be assessed.
Consideration for clinical applications
Although massive studies have resulted in different membrane-coated NP formulations, little research has progressed to clinical practice. This section focuses on the challenges in the clinical translation of CMC-NPs and tries to provide reliable solutions.
First, the in vivo mechanisms of both hybrid and monotypic CMC-NPs remain unknown. One of the main reasons why it is challenging to perform clinical trials for membrane biomimetic carriers is the intricacy and unpredictability of the intermediate process results in vivo. It is risky to assume that the CMC or HCMN would deliver drugs via the theoretical route after entering the human body. To apply membrane coatings beyond the current black box approach [8], researchers need to elucidate more physiological mechanisms, such as internalized mechanisms, intracellular release mechanisms, and subcellular-level actions. This requires a more fundamental understanding of cell biology, which is becoming more prevalent. Therefore, it is imperative to study the in vivo mechanism of membrane biomimetic carrier DDSs, their route of delivery, and their process as soon as possible.
Second, there are issues related to actual benefits. In vivo and in vitro experiments on various types of HCMNs have revealed that HCMNs can indeed exhibit the functional advantages of both types of CMC-NPs. Several experiments, however, have revealed that the mixed benefits of HCMNs are not as high as the unique benefits of monotypic cell membranes in terms of certain functions, such as targeting ability [86, 87] or prolonged blood circulation time [8, 27]. In other words, while the new HCMN DDS verifies and realizes the possibility of 1 + 1, this does not make it > 2.
Third, technical difficulties in acquiring source materials still exist. While cell membranes can be autologous, it may be more practical to obtain and store materials from types of matched donors [24]. However, heterologous cells may have toxicity, biological incompatibility, and immunogenicity. The optimization of protocols to remove unnecessary proteins and retain necessary ones remains to be explored. In addition, changes in membrane protein contents during storage remain another challenge [187, 188]. However, we believe that once a patient-specific cell membrane becomes available, precision medicine will dramatically advance. Addressing disease heterogeneity and establishing personalized therapeutics will then become an achievable goal.
Furthermore, cell membrane-coated platforms will encounter greater developmental opportunities through the integration of newer branches of science and biotechnology (e.g., synthetic biology and biomaterial science), leading to richer therapeutic possibilities. For instance, the use of CMC-NPs to develop vaccines is a novel method for the prevention and treatment of COVID-19, which has been continuously developed and transformed in recent years [189]. Moreover, a few studies have used the membrane from genetically engineered source cells. In these studies, the expression of specific surface markers has been induced or upregulated, optimizing the functionality for a given application [41, 190]. Although cell membranes are by far the main source of membrane coatings, more consideration could be given to other membrane sources, like organelle membranes [42].
Conclusion
Monotypic cell membrane coating or hybrid cell membrane coating confers unique biological properties to NPs, including immune escape, long circulation time, and targeted delivery, thereby enabling more efficient drug delivery. Consequently, cell membrane-coated DDSs have gradually become a novel research hotspot. However, more efforts are needed for the clinical transformation and application of CMC-NPs. Obstacles to the standard protocol, quality control, and large-scale production need to be overcome. Assessment of the mechanism and in vivo process will also guide further improvements in the design and preparation of biomimetic carriers.
Availability of data and materials
Not applicable.
Abbreviations
- DDSs:
-
Drug delivery systems
- NP:
-
Nanoparticle
- EPR:
-
Enhanced permeability and retention
- RES:
-
Reticuloendothelial system
- ABC:
-
Accelerated blood clearance phenomenon
- PEG:
-
Polyethylene glycol
- GRAS:
-
Generally recognized as safe
- FDA:
-
Food and Drug Administration
- CMC-NPs:
-
Cell membrane-coated nanoparticles
- RBC:
-
Red blood cell
- HCMNs:
-
Hybrid cell membrane-coated nanoparticles
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PTT:
-
Photothermal therapy
- SPIO:
-
Superparamagnetic iron oxide
- PDT:
-
Photodynamic therapy
- MRI:
-
Magnetic resonance imaging
- RBC-MNs:
-
RBC membrane-capped magnetic nanoparticles
- NPID:
-
Noninvasive pregnant diagnostics
- WB:
-
Western blot
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TEM:
-
Transmission electron microscopy
- FRET:
-
Förster resonance energy transfer
- CD47:
-
A cluster of differentiated 47
- SIRPα:
-
Signal-regulatory protein alpha
- DAF:
-
Decay-accelerating factor
- CR1:
-
Complement receptor 1
- DOX:
-
Doxorubicin
- SGNPs:
-
Supramolecular gelatin nanoparticles
- Ru–SeNPs:
-
Ru complex-modified selenium nanoparticles
- PMNPs:
-
Platelet membrane-coated nanoparticles
- ICG:
-
Indocyanine green
- CCNPs:
-
Cancer cell membrane-coated nanoparticles
- CCAMs:
-
Cancer cell adhesion molecules
- TF-Ag:
-
Thomsen–Friedenreich glycoantigen
- Ig-SF:
-
Immunoglobulin superfamily
- MNPs:
-
Macrophage membrane-coated nanoparticles
- LPS:
-
Lipopolysaccharide
- PRR:
-
Pattern recognition receptor
- ICB:
-
Immune checkpoint blockade
- EpCAM:
-
Epithelial cell adhesion molecule
- PFTs:
-
Pore-forming toxins
- LCM:
-
Leukocyte–cancer cell HCMN
- CTCs:
-
Circulating tumor cells
- DLS:
-
Dynamic light scattering
- GMP:
-
Good manufacturing practice
References
Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules. 2019;25:112.
Gao Z, Zhang L, Sun Y. Nanotechnology applied to overcome tumor drug resistance. J Controlled Release. 2012;162:45–55.
Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10:7738–48.
Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17:613–20.
Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566–75.
Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C. 2019;98:1252–76.
Wu H, Jiang X, Li Y, Zhou Y, Zhang T, Zhi P, et al. Engineering stem cell derived biomimetic vesicles for versatility and effective targeted delivery. Adv Funct Mater. 2020;30:2006169.
Fang RH, Kroll AV, Gao W. Zhang L. c. Adv Mater. 2018;30:1706759.
Chen L, Hong W, Ren W, Xu T, Qian Z, He Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct Target Ther. 2021;6:225.
Karmali PP, Simberg D. Interactions of nanoparticles with plasma proteins: implication on clearance and toxicity of drug delivery systems. Expert Opin Drug Deliv. 2011;8:343–57.
Tsoi KM, MacParland SA, Ma X-Z, Spetzler VN, Echeverri J, Ouyang B, et al. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15:1212–21.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51.
Yu H, Tang Z, Zhang D, Song W, Zhang Y, Yang Y, et al. Pharmacokinetics, biodistribution and in vivo efficacy of cisplatin loaded poly(l-glutamic acid)-g-methoxy poly(ethylene glycol) complex nanoparticles for tumor therapy. J Controlled Release. 2015;205:89–97.
Zhou H, Fan Z, Deng J, Lemons PK, Arhontoulis DC, Bowne WB, et al. Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016;16:3268–77.
Pannuzzo M, Esposito S, Wu L-P, Key J, Aryal S, Celia C, et al. Overcoming nanoparticle-mediated complement activation by surface PEG pairing. Nano Lett. 2020;20:4312–21.
Zhang P, Sun F, Liu S, Jiang S. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J Controlled Release. 2016;244:184–93.
Mohamed M, Abu Lila AS, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA, et al. PEGylated liposomes: immunological responses. Sci Technol Adv Mater. 2019;20:710–24.
Wang M, **n Y, Cao H, Li W, Hua Y, Webster TJ, et al. Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery. Biomater Sci. 2021;9:1088–103.
Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther. 2000;292:1071–9.
Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci. 2011;108:10980–5.
Hu C-MJ, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–21.
Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–8.
Xuan M, Shao J, Dai L, He Q, Li J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv Healthc Mater. 2015;4:1645–52.
Chugh V, Vijaya Krishna K, Pandit A. Cell membrane-coated mimics: a methodological approach for fabrication, characterization for therapeutic applications, and challenges for clinical translation. ACS Nano. 2021;15:17080–123.
Chen H-Y, Deng J, Wang Y, Wu C-Q, Li X, Dai H-W. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13.
Liao Y, Zhang Y, Blum NT, Lin J, Huang P. Biomimetic hybrid membrane-based nanoplatforms: synthesis, properties and biomedical applications. Nanoscale Horiz. 2020;5:1293–302.
Wang D, Dong H, Li M, Cao Y, Yang F, Zhang K, et al. Erythrocyte–cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12:5241–52.
He H, Guo C, Wang J, Korzun WJ, Wang X-Y, Ghosh S, et al. Leutusome: a biomimetic nanoplatform integrating plasma membrane components of leukocytes and tumor cells for remarkably enhanced solid tumor homing. Nano Lett. 2018;18:6164–74.
Wang D, Liu C, You S, Zhang K, Li M, Cao Y, et al. Bacterial vesicle-cancer cell hybrid membrane-coated nanoparticles for tumor specific immune activation and photothermal therapy. ACS Appl Mater Interfaces. 2020;12:41138–47.
Li Y, Che J, Chang L, Guo M, Bao X, Mu D, et al. CD47- and Integrin α 4/ β 1-comodified-macrophage-membrane-coated nanoparticles enable delivery of colchicine to atherosclerotic plaque. Adv Healthc Mater. 2022;11:2101788.
Liu Y, Luo J, Chen X, Liu W, Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nano-Micro Lett. 2019;11:100.
Rao L, Bu L-L, Xu J-H, Cai B, Yu G-T, Yu X, et al. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. 2015;11:6225–36.
Wang Y, Zhang K, Qin X, Li T, Qiu J, Yin T, et al. Biomimetic nanotherapies: red blood cell based core–shell structured nanocomplexes for atherosclerosis management. Adv Sci. 2019;6:1900172.
Wei X, Gao J, Fang RH, Luk BT, Kroll AV, Dehaini D, et al. Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia. Biomaterials. 2016;111:116–23.
Li J, Ai Y, Wang L, Bu P, Sharkey CC, Wu Q, et al. Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials. 2016;76:52–65.
McMillan JR, Watson IA, Ali M, Jaafar W. Evaluation and comparison of algal cell disruption methods: microwave, waterbath, blender, ultrasonic and laser treatment. Appl Energy. 2013;103:128–34.
Kuznetsov VI, Haws SA, Fox CA, Denu JM. General method for rapid purification of native chromatin fragments. J Biol Chem. 2018;293:12271–82.
Tam YJ, Allaudin ZN, Lila MAM, Bahaman AR, Tan JS, Rezaei MA. Enhanced cell disruption strategy in the release of recombinant hepatitis B surface antigen from Pichia pastoris using response surface methodology. BMC Biotechnol. 2012;12:70.
van Hee P, Middelberg APJ, van der Lans RGJM, van der Wielen LAM. Relation between cell disruption conditions, cell debris particle size, and inclusion body release. Biotechnol Bioeng. 2004;88:100–10.
Kang T, Zhu Q, Wei D, Feng J, Yao J, Jiang T, et al. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11:1397–411.
Park JH, Jiang Y, Zhou J, Gong H, Mohapatra A, Heo J, et al. Genetically engineered cell membrane–coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci Adv. 2021;7:eabf7820.
Gong H, Zhang Q, Komarla A, Wang S, Duan Y, Zhou Z, et al. Nanomaterial biointerfacing via mitochondrial membrane coating for targeted detoxification and molecular detection. Nano Lett. 2021;21:2603–9.
Deng G, Sun Z, Li S, Peng X, Li W, Zhou L, et al. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12:12096–108.
Nie D, Dai Z, Li J, Yang Y, ** Z, Wang J, et al. Cancer-cell-membrane-coated nanoparticles with a yolk–shell structure augment cancer chemotherapy. Nano Lett. 2020;20:936–46.
Franke WW, Lüder MR, Kartenbeck J, Zerban H, Keenan TW. Involvement of vesicle coat material in casein secretion and surface regeneration. J Cell Biol. 1976;69:173–95.
Javed S, Alshehri S, Shoaib A, Ahsan W, Sultan MH, Alqahtani SS, et al. Chronicles of nanoerythrosomes: an erythrocyte-based biomimetic smart drug delivery system as a therapeutic and diagnostic tool in cancer therapy. Pharmaceutics. 2021;13:368.
Harrison STL. Cell disruption. Compr Biotechnol. Elsevier; 2011 [cited 2022 Jun 28]. p. 619–40. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780080885049001276
Hankins NP, Singhv R. Emerging membrane technology for sustainable water treatment. Elsevier Science 2016.
Danaeifar M. New horizons in develo** cell lysis methods: a review. Biotechnol Bioeng. 2022;bit.28198.
Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238-IN27.
Rao L, Bu L-L, Cai B, Xu J-H, Li A, Zhang W-F, et al. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv Mater. 2016;28:3460–6.
Dehaini D, Wei X, Fang RH, Masson S, Angsantikul P, Luk BT, et al. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv Mater. 2017;29:1606209.
Zhou M, Lai W, Li G, Wang F, Liu W, Liao J, et al. Platelet membrane-coated and VAR2CSA malaria protein-functionalized nanoparticles for targeted treatment of primary and metastatic cancer. ACS Appl Mater Interfaces. 2021;13:25635–48.
Chen Z, Zhao P, Luo Z, Zheng M, Tian H, Gong P, et al. Cancer cell membrane–biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10:10049–57.
Thamphiwatana S, Angsantikul P, Escajadillo T, Zhang Q, Olson J, Luk BT, et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci. 2017;114:11488–93.
Tang J, Shen D, Caranasos TG, Wang Z, Vandergriff AC, Allen TA, et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun. 2017;8:13724.
Pitchaimani A, Nguyen TDT, Aryal S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials. 2018;160:124–37.
Jiang L, Zhu Y, Luan P, Xu J, Ru G, Fu J-G, et al. Bacteria - anchoring hybrid liposome capable of absorbing multiple toxins for antivirulence therapy of Escherichia coli infection. ACS Nano. 2021;15:4173–85.
Johnston MJW, Semple SC, Klimuk SK, Ansell S, Maurer N, Cullis PR. Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim Biophys Acta BBA - Biomembr. 2007;1768:1121–7.
Niu X, Chen J, Gao J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: focus on recent advances. Asian J Pharm Sci. 2019;14:480–96.
Chen Z-J, Yang S-C, Liu X-L, Gao Y, Dong X, Lai X, et al. Nanobowl-supported liposomes improve drug loading and delivery. Nano Lett. 2020;20:4177–87.
Anwekar H, Patel S, Singhai AK. Liposome-as drug carriers. Int J Pharm Life Sci. 2011.
Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116:2826–85.
Meng Q-F, Rao L, Zan M, Chen M, Yu G-T, Wei X, et al. Macrophage membrane-coated iron oxide nanoparticles for enhanced photothermal tumor therapy. Nanotechnology. 2018;29: 134004.
Lai P-Y, Huang R-Y, Lin S-Y, Lin Y-H, Chang C-W. Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv. 2015;5:98222–30.
Karlsson HL, Gustafsson J, Cronholm P, Möller L. Size-dependent toxicity of metal oxide particles—a comparison between nano- and micrometer size. Toxicol Lett. 2009;188:112–8.
Ahamed M, Siddiqui MA, Akhtar MJ, Ahmad I, Pant AB, Alhadlaq HA. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem Biophys Res Commun. 2010;396:578–83.
Naz S, Gul A, Zia M. Toxicity of copper oxide nanoparticles: a review study. IET Nanobiotechnol. 2020;14:1–13.
Sun D, Gong L, **e J, Gu X, Li Y, Cao Q, et al. Toxicity of silicon dioxide nanoparticles with varying sizes on the cornea and protein corona as a strategy for therapy. Sci Bull. 2018;63:907–16.
Guo P, Huang J, Zhao Y, Martin CR, Zare RN, Moses MA. Nanomaterial preparation by extrusion through nanoporous membranes. Small. 2018;14:1703493.
Palmgren MG, Askerlund P, Fredrikson K, Widell S, Sommarin M, Larsson C. Sealed inside-out and right-side-out plasma membrane vesicles: optimal conditions for formation and separation. Plant Physiol. 1990;92:871–80.
Luk BT, Jack Hu C-M, Fang RH, Dehaini D, Carpenter C, Gao W, et al. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale. 2013;6:2730–7.
György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88.
Copp JA, Fang RH, Luk BT, Hu C-MJ, Gao W, Zhang K, et al. Clearance of pathological antibodies using biomimetic nanoparticles. Proc Natl Acad Sci. 2014;111:13481–6.
He W, Frueh J, Wu Z, He Q. Leucocyte membrane-coated janus microcapsules for enhanced photothermal cancer treatment. langmuir. 2016;32:3637–44.
Arrigo R, Teresi R, Gambarotti C, Parisi F, Lazzara G, Dintcheva N. Sonication-induced modification of carbon nanotubes: effect on the rheological and thermo-oxidative behaviour of polymer-based nanocomposites. Materials. 2018;11:383.
Rennhofer H, Zanghellini B. Dispersion state and damage of carbon nanotubes and carbon nanofibers by ultrasonic dispersion: a review. Nanomaterials. 2021;11:1469.
Rao L, Cai B, Bu L-L, Liao Q-Q, Guo S-S, Zhao X-Z, et al. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11:3496–505.
Kim K, Lee WG. Electroporation for nanomedicine: a review. J Mater Chem B. 2017;5:2726–38.
Ottonelli I, Duskey JT, Rinaldi A, Grazioli MV, Parmeggiani I, Vandelli MA, et al. Microfluidic technology for the production of hybrid nanomedicines. Pharmaceutics. 2021;13:1495.
Holtze C. Large-scale droplet production in microfluidic devices—an industrial perspective. J Phys Appl Phys. 2013;46: 114008.
Scott-Taylor TH, Pettengell R, Clarke I, Stuhler G, La Barthe MC, Walden P, et al. Human tumour and dendritic cell hybrids generated by electrofusion: potential for cancer vaccines. Biochim Biophys Acta BBA - Mol Basis Dis. 2000;1500:265–79.
Ramos C, Bonenfant D, Teissie J. Cell hybridization by electrofusion on filters. Anal Biochem. 2002;302:213–9.
Liu W-L, Zou M-Z, Liu T, Zeng J-Y, Li X, Yu W-Y, et al. Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells. Nat Commun. 2019;10:3199.
Koido S, Homma S, Okamoto M, Namiki Y, Takakura K, Takahara A, et al. Combined TLR2/4-activated dendritic/tumor cell fusions induce augmented cytotoxic T lymphocytes. Arens R, editor. PLoS One. 2013;8:e59280.
Jiang Q, Liu Y, Guo R, Yao X, Sung S, Pang Z, et al. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308.
Gong C, Yu X, You B, Wu Y, Wang R, Han L, et al. Macrophage-cancer hybrid membrane-coated nanoparticles for targeting lung metastasis in breast cancer therapy. J Nanobiotechnology. 2020;18:92.
Lawrie WC, Desmond JA, Spence D, Anderson S, Edmondson C. Determination of radium-226 in environmental and personal monitoring samples. Appl Radiat Isot. 2000;53:133–7.
Wang Z, Cheng L, Sun Y, Wei X, Cai B, Liao L, et al. Enhanced isolation of fetal nucleated red blood cells by enythrocyte-leukocyte hybrid membrane-coated magnetic nanoparticles for noninvasive pregnant diagnostics. Anal Chem. 2021;93:1033–42.
Wang Y, Luan Z, Zhao C, Bai C, Yang K. Target delivery selective CSF-1R inhibitor to tumor-associated macrophages via erythrocyte-cancer cell hybrid membrane camouflaged pH-responsive copolymer micelle for cancer immunotherapy. Eur J Pharm Sci. 2020;142: 105136.
Liu Y, Wang X, Ouyang B, Liu X, Du Y, Cai X, et al. Erythrocyte–platelet hybrid membranes coating polypyrrol nanoparticles for enhanced delivery and photothermal therapy. J Mater Chem B. 2018;6:7033–41.
Li M, Xu Z, Zhang L, Cui M, Zhu M, Guo Y, et al. Targeted noninvasive treatment of choroidal neovascularization by hybrid cell-membrane-cloaked biomimetic nanoparticles. ACS Nano. 2021;15:9808–19.
Hou W, Ma D, He X, Han W, Ma J, Wang H, et al. Subnanometer-precision measurements of transmembrane motions of biomolecules in plasma membranes using quenchers in extracellular environment. Nano Lett. 2021;21:485–91.
Lejeune A, Moorjani M, Gicquaud C, Lacroix J, Poyet P, Gaudreault R. Nanoerythrosome, a new derivative of erythrocyte ghost: preparation and antineoplastic potential as drug carrier for daunorubicin. Anticancer Res. 1994;14:915–9.
Fu Q, Lv P, Chen Z, Ni D, Zhang L, Yue H, et al. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane. Nanoscale. 2015;7:4020–30.
Luk BT, Fang RH, Hu C-MJ, Copp JA, Thamphiwatana S, Dehaini D, et al. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics. 2016;6:1004–11.
Chai Z, Hu X, Wei X, Zhan C, Lu L, Jiang K, et al. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J Controlled Release. 2017;264:102–11.
Lin A, Liu Y, Zhu X, Chen X, Liu J, Zhou Y, et al. Bacteria-responsive biomimetic selenium nanosystem for multidrug-resistant bacterial infection detection and inhibition. ACS Nano. 2019;13:13965–84.
Zhang Y, Gao W, Chen Y, Escajadillo T, Ungerleider J, Fang RH, et al. Self-assembled colloidal gel using cell membrane-coated nanosponges as building blocks. ACS Nano. 2017;11:11923–30.
Zhang C, Zhang P-Q, Guo S, Chen G, Zhao Z, Wang G-X, et al. Application of biomimetic cell-derived nanoparticles with mannose modification as a novel vaccine delivery platform against teleost fish viral disease. ACS Biomater Sci Eng. 2020;6:6770–7.
Gao W, Hu C-MJ, Fang RH, Luk BT, Su J, Zhang L. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater. 2013;25:3549–53.
Piao J-G, Wang L, Gao F, You Y-Z, **ong Y, Yang L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8:10414–25.
Bosman GJCGM. Survival of red blood cells after transfusion: processes and consequences. Front Physiol [Internet]. 2013 [cited 2022 Jun 28];4. Available from: http://journal.frontiersin.org/article/10.3389/fphys.2013.00376/abstract
Oldenborg P-A, Zheleznyak A, Fang Y-F, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4.
Zhang W, Huang Q, **ao W, Zhao Y, Pi J, Xu H, et al. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front Immunol. 2020;11:18.
McCracken MN, Cha AC, Weissman IL. Molecular pathways: activating T cells after cancer cell phagocytosis from blockade of CD47 “Don’t Eat Me” signals. Clin Cancer Res. 2015;21:3597–601.
First Pavlov State Medical University of St. Peterburg, Galkin M. Application of cellular and artificial membranes in nanomedicine. Vestn St Petersburg Univ Med. 2020;15:290–9.
Fang RH, Hu C-MJ, Zhang L. Nanoparticles disguised as red blood cells to evade the immune system. Expert Opin Biol Ther. 2012;12:385–9.
**a Q, Zhang Y, Li Z, Hou X, Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9:675–89.
Ben-Akiva E, Meyer RA, Yu H, Smith JT, Pardoll DM, Green JJ. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci Adv. 2020;6:eaay9035.
Nel A, **a T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–7.
Li L-L, Xu J-H, Qi G-B, Zhao X, Yu F, Wang H. Core–shell supramolecular gelatin nanoparticles for adaptive and “on-demand” antibiotic delivery. ACS Nano. 2014;8:4975–83.
Godfrin Y, Horand F, Franco R, Dufour E, Kosenko E, Bax BE, et al. International seminar on the red blood cells as vehicles for drugs. Expert Opin Biol Ther. 2012;12:127–33.
Vincy A, Mazumder S, Amrita null, Banerjee I, Hwang KC, Vankayala R. Recent progress in red blood cells-derived particles as novel bioinspired drug delivery systems: challenges and strategies for clinical translation. Front Chem. 2022;10:905256.
Magnani M. Erythrocytes as carriers for drugs: the transition from the laboratory to the clinic is approaching. Expert Opin Biol Ther. 2012;12:137–8.
Gao W, Zhang L. Engineering red-blood-cell-membrane-coated nanoparticles for broad biomedical applications. AIChE J. 2015;61:738–46.
Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, Tian LL, et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano. 2014;8:11243–53.
Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006;4:445–57.
Kieffer N, Phillips DR. Platelet membrane glycoproteins: functions in cellular interactions. Annu Rev Cell Biol. 1990;6:329–57.
Modery-Pawlowski CL, Tian LL, Ravikumar M, Wong TL, Gupta AS. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials. 2013;34:3031–41.
Broos K, De Meyer SF, Feys HB, Vanhoorelbeke K, Deckmyn H. Blood platelet biochemistry. Thromb Res. 2012;129:245–9.
Sekhon UDS, Swingle K, Girish A, Luc N, de la Fuente M, Alvikas J, et al. Platelet-mimicking procoagulant nanoparticles augment hemostasis in animal models of bleeding. Sci Transl Med. 2022;14:eabb8975.
Jurk K, Kehrel BE. Platelets: physiology and biochemistry. Semin Thromb Hemost. 2005;31:381–92.
Payrastre B, Missy K, Trumel C, Bodin S, Plantavid M, Chap H. The integrin αIIb/β3 in human platelet signal transduction. Biochem Pharmacol. 2000;60:1069–74.
He Y, Li R, Liang J, Zhu Y, Zhang S, Zheng Z, et al. Drug targeting through platelet membrane-coated nanoparticles for the treatment of rheumatoid arthritis. Nano Res. 2018;11:6086–101.
Hamilos M, Petousis S, Parthenakis F. Interaction between platelets and endothelium: from pathophysiology to new therapeutic options. Cardiovasc Diagn Ther. 2018;8:568–80.
Lavergne M, Janus-Bell E, Schaff M, Gachet C, Mangin P. Platelet integrins in tumor metastasis: do they represent a therapeutic target? Cancers. 2017;9:133.
Ye H, Wang K, Wang M, Liu R, Song H, Li N, et al. Bioinspired nanoplatelets for chemo-photothermal therapy of breast cancer metastasis inhibition. Biomaterials. 2019;206:1–12.
Han H, Bártolo R, Li J, Shahbazi M-A, Santos HA. Biomimetic platelet membrane-coated nanoparticles for targeted therapy. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft Pharm Verfahrenstechnik EV. 2022;172:1–15.
Zhu J-Y, Zheng D-W, Zhang M-K, Yu W-Y, Qiu W-X, Hu J-J, et al. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16:5895–901.
Sick E, Jeanne A, Schneider C, Dedieu S, Takeda K, Martiny L. CD47 update: a multifaceted actor in the tumour microenvironment of potential therapeutic interest: CD47 in the tumour microenvironment. Br J Pharmacol. 2012;167:1415–30.
Bose RJ, Paulmurugan R, Moon J, Lee S-H, Park H. Cell membrane-coated nanocarriers: the emerging targeted delivery system for cancer theranostics. Drug Discov Today. 2018;23:891–9.
Bellone S, Siegel ER, Cocco E, Cargnelutti M, Silasi D-A, Azodi M, et al. Overexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer: implications for epithelial cell adhesion molecule-specific immunotherapy. Int J Gynecol Cancer. 2009;19:860–6.
Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–8.
Rabiee N, Yaraki MT, Garakani SM, Garakani SM, Ahmadi S, Lajevardi A, et al. Recent advances in porphyrin-based nanocomposites for effective targeted imaging and therapy. Biomaterials. 2020;232: 119707.
McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase – essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–32.
Lian M, Shao S, Liu M, Shi Y, Zhang H, Chen D. Cell membrane-coated nanoparticles as peroxidase mimetics for cancer cell targeted detection and therapy. Talanta. 2022;238: 123071.
Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, et al. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv Mater. 2016;28:9581–8.
Yang J, Teng Y, Fu Y, Zhang C. Chlorins e6 loaded silica nanoparticles coated with gastric cancer cell membrane for tumor specific photodynamic therapy of gastric cancer. Int J Nanomedicine. 2019;14:5061–71.
** J, Krishnamachary B, Barnett JD, Chatterjee S, Chang D, Mironchik Y, et al. Human cancer cell membrane-coated biomimetic nanoparticles reduce fibroblast-mediated invasion and metastasis and induce T-cells. ACS Appl Mater Interfaces. 2019;11:7850–61.
Fontana F, Shahbazi M-A, Liu D, Zhang H, Mäkilä E, Salonen J, et al. Multistaged nanovaccines based on porous silicon@acetalated dextran@cancer cell membrane for cancer immunotherapy. Adv Mater. 2017;29:1603239.
Pereira-Silva M, Santos AC, Conde J, Hoskins C, Concheiro A, Alvarez-Lorenzo C, et al. Biomimetic cancer cell membrane-coated nanosystems as next-generation cancer therapies. Expert Opin Drug Deliv. 2020;17:1515–8.
Gong X, Li J, Tan T, Wang Z, Wang H, Wang Y, et al. Emerging approaches of cell-based nanosystems to target cancer metastasis. Adv Funct Mater. 2019;29:1903441.
Harris JC, Scully MA, Day ES. Cancer cell membrane-coated nanoparticles for cancer management. Cancers. 2019;11:1836.
Zou M-Z, Li Z-H, Bai X-F, Liu C-J, Zhang X-Z. Hybrid vesicles based on autologous tumor cell membrane and bacterial outer membrane to enhance innate immune response and personalized tumor immunotherapy. Nano Lett. 2021;21:8609–18.
**ong X, Zhao J, Pan J, Liu C, Guo X, Zhou S. Personalized nanovaccine coated with calcinetin-expressed cancer cell membrane antigen for cancer immunotherapy. Nano lett. 2021;21:8418–25.
Si J, Shao S, Shen Y, Wang K. Macrophages as active nanocarriers for targeted early and adjuvant cancer chemotherapy. Small. 2016;12:5108–19.
Cai H, Wang R, Guo X, Song M, Yan F, Ji B, et al. Combining gemcitabine-loaded macrophage-like nanoparticles and erlotinib for pancreatic cancer therapy. Mol Pharm. 2021;18:2495–506.
Xuan M, Shao J, Dai L, Li J, He Q. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8:9610–8.
Chen C, Song M, Du Y, Yu Y, Li C, Han Y, et al. Tumor-associated-macrophage-membrane-coated nanoparticles for improved photodynamic immunotherapy. Nano Lett. 2021;21:5522–31.
Rao L, He Z, Meng Q-F, Zhou Z, Bu L-L, Guo S-S, et al. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles: Effective cancer targeting and imaging. J Biomed Mater Res A. 2017;105:521–30.
Poudel K, Banstola A, Gautam M, Soe Z, Phung CD, Pham LM, et al. Macrophage-membrane-camouflaged disintegrable and excretable nanoconstruct for deep tumor penetration. ACS Appl Mater Interfaces. 2020;12:56767–81.
Louwe PA, Badiola Gomez L, Webster H, Perona-Wright G, Bain CC, Forbes SJ, et al. Recruited macrophages that colonize the post-inflammatory peritoneal niche convert into functionally divergent resident cells. Nat Commun. 2021;12:1770.
Li R, He Y, Zhu Y, Jiang L, Zhang S, Qin J, et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19:124–34.
Shen S, Han F, Yuan A, Wu L, Cao J, Qian J, et al. Engineered nanoparticles disguised as macrophages for trap** lipopolysaccharide and preventing endotoxemia. Biomaterials. 2019;189:60–8.
Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34:392–7.
Jahromi LP, Shahbazi M, Maleki A, Azadi A, Santos HA. Chemically engineered immune cell-derived microrobots and biomimetic nanoparticles: emerging biodiagnostic and therapeutic tools. Adv Sci. 2021;8:2002499.
Lassenius MI, Pietiläinen KH, Kaartinen K, Pussinen PJ, Syrjänen J, Forsblom C, et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34:1809–15.
Cao B, Yang M, Zhu Y, Qu X, Mao C. Stem cells loaded with nanoparticles as a drug carrier for in vivo breast cancer therapy. Adv Mater. 2014;26:4627–31.
Wu M, Le W, Mei T, Wang Y, Chen B, Liu Z, et al. Cell membrane camouflaged nanoparticles: a new biomimetic platform for cancer photothermal therapy. Int J Nanomedicine. 2019;14:4431–48.
Huang Y, Gao X, Chen J. Leukocyte-derived biomimetic nanoparticulate drug delivery systems for cancer therapy. Acta Pharm Sin B. 2018;8:4–13.
Zhang L, Li R, Chen H, Wei J, Qian H, Su S, et al. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer. Int J Nanomedicine. 2017;12:2129–42.
Tang H, Xue Y, Li B, Xu X, Zhang F, Guo J, et al. Membrane-camouflaged supramolecular nanoparticles for co-delivery of chemotherapeutic and molecular-targeted drugs with siRNA against patient-derived pancreatic carcinoma. Acta Pharm Sin B. 2022;12:3410–26.
Hu C-MJ, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8:336–40.
Ma J, Jiang L, Liu G. Cell membrane-coated nanoparticles for the treatment of bacterial infection. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;e1825.
Chen Y, Chen M, Zhang Y, Lee JH, Escajadillo T, Gong H, et al. Broad-spectrum neutralization of pore-forming toxins with human erythrocyte membrane-coated nanosponges. Adv Healthc Mater. 2018;7: e1701366.
Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L, et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13:1182–90.
Zeng Y, Li S, Zhang S, Wang L, Yuan H, Hu F. Cell membrane coated-nanoparticles for cancer immunotherapy. Acta Pharm Sin B. 2022;12:3233–54.
Anwar M, Muhammad F, Akhtar B, Anwar MI, Raza A, Aleem A. Outer membrane protein-coated nanoparticles as antibacterial vaccine candidates. Int J Pept Res Ther. 2021;27:1689–97.
Fang RH, Hu C-MJ, Chen KNH, Luk BT, Carpenter CW, Gao W, et al. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale. 2013;5:8884.
Ding C, Zhang C, Cheng S, **an Y. Multivalent aptamer functionalized Ag 2 S nanodots/hybrid cell membrane-coated magnetic nanobioprobe for the ultrasensitive isolation and detection of circulating tumor cells. Adv Funct Mater. 2020;30:1909781.
Esteban-Fernández de Ávila B, Angsantikul P, Ramírez-Herrera DE, Soto F, Teymourian H, Dehaini D, et al. Hybrid biomembrane–functionalized nanorobots for concurrent removal of pathogenic bacteria and toxins. Sci Robot. 2018;3:eaat0485.
Wang G, Chen X, Liu S, Wong C, Chu S. Mechanical chameleon through dynamic real-time plasmonic tuning. ACS Nano. 2016;10:1788–94.
Han X, Shen S, Fan Q, Chen G, Archibong E, Dotti G, et al. Red blood cell–derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci Adv. 2019;5:eaaw6870.
Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114:10869–939.
**ong J, Wu M, Chen J, Liu Y, Chen Y, Fan G, et al. Cancer-erythrocyte hybrid membrane-camouflaged magnetic nanoparticles with enhanced photothermal-immunotherapy for ovarian cancer. ACS Nano. 2021;15:19756–70.
Chen Long, Qin Hao, Zhao Ruifang, Zhao **ao, Lin Liangru, Chen Yang, et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci Transl Med. American Association for the Advancement of Science; 2021;13:eabc2816.
Wang L, Asghar W, Demirci U, Wan Y. Nanostructured substrates for isolation of circulating tumor cells. Nano Today. 2013;8:374–87.
Committee on Standards, and American Association of Blood Banks. Standards Program Committee. FACT-JACIE international standards for hematopoietic cellular therapy product collection, processing, and administration. Stand. Blood Banks Transfus. Serv. 1974.
Guidelines for Quality Control and Preclinical Research of Stem Cell Preparations (Trial). 2015.
Xu W-J, Cai J-X, Li Y-J, Wu J-Y, **ang D. Recent progress of macrophage vesicle-based drug delivery systems. Drug Deliv Transl Res [Internet]. 2022 [cited 2022 Jun 28]; Available from: https://springer.longhoe.net/10.1007/s13346-021-01110-5.
Malhotra S, Dumoga S, Singh N. Red blood cells membrane‐derived nanoparticles: applications and key challenges in their clinical translation. WIREs Nanomed Nanobiotechnol. 2022 [cited 2022 Sep 14];14. Available from: https://onlinelibrary.wiley.com/doi/10.1002/wnan.1776.
Vetten MA, Yah CS, Singh T, Gulumian M. Challenges facing sterilization and depyrogenation of nanoparticles: effects on structural stability and biomedical applications. Nanomedicine Nanotechnol Biol Med. 2014;10:1391–9.
Alphandéry E. A discussion on existing nanomedicine regulation: progress and pitfalls. Appl Mater Today. 2019;17:193–205.
Merivaara A, Zini J, Koivunotko E, Valkonen S, Korhonen O, Fernandes FM, et al. Preservation of biomaterials and cells by freeze-drying: change of paradigm. J Controlled Release. 2021;336:480–98.
Ragelle H, Danhier F, Préat V, Langer R, Anderson DG. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert Opin Drug Deliv. 2017;14:851–64.
Stewart A, Urbaniak S, Turner M, Bessos H. The application of a new quantitative assay for the monitoring of integrin-associated protein CD47 on red blood cells during storage and comparison with the expression of CD47 and phosphatidylserine with flow cytometry. Transfusion (Paris). 2005;45:1496–503.
Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfusion (Paris). 2008;48:1943–53.
Pereira-Silva M, Chauhan G, Shin MD, Hoskins C, Madou MJ, Martinez-Chapa SO, et al. Unleashing the potential of cell membrane-based nanoparticles for COVID-19 treatment and vaccination. Expert Opin Drug Deliv. 2021;18:1395–414.
Jiang Y, Krishnan N, Zhou J, Chekuri S, Wei X, Kroll AV, et al. Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity. Adv Mater. 2020;32:2001808.
Funding
This work was supported by the National Natural Science Foundation of China (U22A20383, 81620108028), the Natural Science Foundation of Zhejiang Province (LD22H300002), and the Fundamental Research Funds for the Central Universities (2021QNA7021).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. **n-Chi Jiang and Jian-Qing Gao planned and structured the review. The first draft and all the illustrations were created by Hui Liu and Yu-Yan Su. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Su, YY., Jiang, XC. et al. Cell membrane-coated nanoparticles: a novel multifunctional biomimetic drug delivery system. Drug Deliv. and Transl. Res. 13, 716–737 (2023). https://doi.org/10.1007/s13346-022-01252-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01252-0