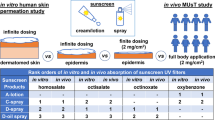
Abstract
Due to high variability during clinical pharmacokinetic (PK) evaluation, the prediction of in vivo exposure from in vitro absorption testing of topical semisolid and liquid dermal products has historically proven difficult. Since absorption from unoccluded formulations can be influenced by environmental factors such as temperature and humidity, maximal effort must be placed on the harmonization of experimental parameters between in vitro and in vivo testing conditions to establish accurate in vitro/in vivo correlations (IVIVC). Using four different sunscreen formulations as a model, we performed in vitro permeation testing (IVPT) studies with excised human skin and maintained strict harmonization techniques to control application time, occlusion, temperature, and humidity during in vivo human serum PK evaluation. The goal was to investigate if increased control over experimental parameters would result in decreased inter-subject variability of common topical formulations leading to acceptable IVIVC establishment. Using a deconvolution-based approach, excellent point-to-point (Level A correlation) IVIVC for the entire 12-h study duration was achieved for all four sunscreen formulations with < 10% prediction error of both area under the curve (AUC) and peak concentration (Cmax) estimation. The low variability of in vivo absorption data presents a proof-of-concept protocol design for testing of complex semisolid and liquid topical formulations applied over a large surface area with reapplication in a reliable manner. This work also presents the opportunity for expanded development of testing for the impact of altered temperature and humidity conditions on product absorption in vivo with a high degree of precision.
Graphical abstract









Similar content being viewed by others
Data availability
N/A.
References
Food and Drug Administration. Guidance for industry: extended release oral dosage forms-development, evaluation, and application of in vitro/in vivo correlations. 1997.
Godin B, Touitou E. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv Drug Deliv Rev. 2007. https://doi.org/10.1016/j.addr.2007.07.004.
Valiveti S, Paudel KS, Hammell DC, Hamad MO, Chen J, Crooks PA, Stinchcomb AL. In vitro/in vivo correlation of transdermal naltrexone prodrugs in hairless guinea pigs. Pharm Res. 2005. https://doi.org/10.1007/s11095-005-4593-0.
Trends in the topical delivery of dermatology medications 2017 [Available from: https://www.manufacturingchemist.com/news/article_page/Trends_in_the_topical_delivery_of_dermatology_medications/129282.
Khire A, Vavia P. Bioavailability, bioequivalence, and in vitro-in vivo correlation of oxybutynin transdermal patch in rabbits. Drug Deliv Transl Res. 2014. https://doi.org/10.1007/s13346-013-0170-y.
Sun L, Cun D, Yuan B, Cui H, ** collective data for demonstrating therapeutic (non)equivalence of topical semisolids: a case study of "ready-to-use" vehicles. Int J Pharm. 2017. https://doi.org/10.1016/j.ijpharm.2017.06.018.
Yacobi A, Shah VP, Bashaw ED, Benfeldt E, Davit B, Ganes D, Ghosh T, Kanfer I, Kasting GB, Katz L, Lionberger R, Lu GW, Maibach HI, Pershing LK, Rackley RJ, Raw A, Shukla CG, Thakker K, Wagner N, Zovko E, Lane ME. Current challenges in bioequivalence, quality, and novel assessment technologies for topical products. Pharm Res. 2014. https://doi.org/10.1007/s11095-013-1259-1.
Bashaw ED, Tran DC, Shukla CG, Liu X. Maximal usage trial: an overview of the design of systemic bioavailability trial for topical dermatological products. Ther Innov Regul Sci. 2015. https://doi.org/10.1177/2168479014539157.
Food and Drug Administration. Maximal usage trials for topically applied active ingredients being considered for inclusion in an over-the-counter monograph: study elements and considerations. 2019.
Yamamoto S, Karashima M, Arai Y, Tohyama K, Amano N. Prediction of human pharmacokinetic profile after transdermal drug application using excised human skin. J Pharm Sci. 2017. https://doi.org/10.1016/j.xphs.2017.03.003.
Akomeah F, Nazir T, Martin GP, Brown MB. Effect of heat on the percutaneous absorption and skin retention of three model penetrants. Eur J Pharm Sci. 2004. https://doi.org/10.1016/j.ejps.2003.10.025.
Hao J, Ghosh P, Li SK, Newman B, Kasting GB, Raney SG. Heat effects on drug delivery across human skin. Expert Opin Drug Deliv. 2016. https://doi.org/10.1517/17425247.2016.1136286.
Park JH, Lee JW, Kim YC, Prausnitz MR. The effect of heat on skin permeability. Int J Pharm. 2008. https://doi.org/10.1016/j.ijpharm.2008.03.032.
Merwe E, Ackermann C. Physical changes in hydrated skin. Int J Cosmet Sci. 1987. https://doi.org/10.1111/j.1467-2494.1987.tb00478.x.
Food and Drug Administration. Transdermal and topical delivery systems - product development and quality considerations. 2019. https://www.fda.gov/media/132674/download.
Food and Drug Administration. Guidance for industry: Q1a(r2) stability testing of new drug substances and products. 2003. https://www.fda.gov/media/71707/download.
Jones K, Cocker J, Dodd LJ, Fraser I. Factors affecting the extent of dermal absorption of solvent vapours: a human volunteer study. Ann Occup Hyg. 2003.https://doi.org/10.1093/annhyg/meg016.
Wolkoff P. Indoor air humidity, air quality, and health - an overview. Int J Hyg Environ Health. 2018. https://doi.org/10.1016/j.ijheh.2018.01.015.
Matta MK, Zusterzeel R, Pilli NR, Patel V, Volpe DA, Florian J, Oh L, Bashaw E, Zineh I, Sanabria C, Kemp S, Godfrey A, Adah S, Coelho S, Wang J, Furlong LA, Ganley C, Michele T, Strauss DG. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019. https://doi.org/10.1001/jama.2019.5586.
Sigma-Aldrich. Oxybenzone safety data sheet. 2021.
Jiang R, Roberts MS, Collins DM, Benson HA. Absorption of sunscreens across human skin: an evaluation of commercial products for children and adults. Br J Clin Pharmacol. 1999. https://doi.org/10.1046/j.1365-2125.1999.00056.x.
Romanhole RC, Fava ALM, Tundisi LL, Macedo LM, Santos EMD, Ataide JA, Mazzola PG. Unplanned absorption of sunscreen ingredients: Impact of formulation and evaluation methods. Int J Pharm. 2020. https://doi.org/10.1016/j.ijpharm.2020.120013.
Frederiksen H, Krause M, Jorgensen N, Rehfeld A, Skakkebaek NE, Andersson AM. Uv filters in matched seminal fluid-, urine-, and serum samples from young men. J Expo Sci Environ Epidemiol. 2021. https://doi.org/10.1038/s41370-020-0209-3.
Benson HA. Assessment and clinical implications of absorption of sunscreens across skin. Am J Clin Dermatol. 200. https://doi.org/10.2165/00128071-200001040-00003.
Gonzalez H, Farbrot A, Larko O, Wennberg AM. Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. Br J Dermatol. 2006. https://doi.org/10.1111/j.1365-2133.2005.07007.x.
Gustavsson Gonzalez H, Farbrot A, Larko O. Percutaneous absorption of benzophenone-3, a common component of topical sunscreens. Clin Exp Dermatol. 2002. https://doi.org/10.1046/j.1365-2230.2002.01095.x.
Hayden CG, Roberts MS, Benson HA. Systemic absorption of sunscreen after topical application. The Lancet. 1997. https://doi.org/10.1016/S0140-6736(05)62032-6.
Janjua NR, Kongshoj B, Andersson AM, Wulf HC. Sunscreens in human plasma and urine after repeated whole-body topical application. J Eur Acad Dermatol Venereol. 2008. https://doi.org/10.1111/j.1468-3083.2007.02492.x.
Matta MK, Florian J, Zusterzeel R, Pilli NR, Patel V, Volpe DA, Yang Y, Oh L, Bashaw E, Zineh I, Sanabria C, Kemp S, Godfrey A, Adah S, Coelho S, Wang J, Furlong LA, Ganley C, Michele T, Strauss DG. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020. https://doi.org/10.1001/jama.2019.20747.
Hiller J, Klotz K, Meyer S, Uter W, Hof K, Greiner A, Goen T, Drexler H. Systemic availability of lipophilic organic uv filters through dermal sunscreen exposure. Environ Int. 2019. https://doi.org/10.1016/j.envint.2019.105068.
Bakardzhiev IG, F. The skin barrier in patients with lichen simplex chronicus. Symbiosis. 2016. https://doi.org/10.15226/2378-1726/3/3/00131.
Food and Drug Administration. Bioanalytical method validation guidance for industry. 2018. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf.
Huh Y, Smith DE, Feng MR. Interspecies scaling and prediction of human clearance: comparison of small- and macro-molecule drugs. Xenobiotica. 2011. https://doi.org/10.3109/00498254.2011.598582.
Fediuk DJ, Wang T, Chen Y, Parkinson FE, Namaka MP, Simons KJ, Burczynski FJ, Gu X. Tissue disposition of the insect repellent deet and the sunscreen oxybenzone following intravenous and topical administration in rats. Biopharm Drug Dispos. 2011. https://doi.org/10.1002/bdd.765.
Flynn GL. Cutaneous and transdermal delivery-processes and systems of delivery. Modern Pharm. 2002;293–363.
Sakore S, Chakraborty B. In vitro–in vivo correlation (ivivc): a strategic tool in drug development. J Bioequivalence Bioavailab. 2011. https://doi.org/10.4172/jbb.S3-001.
Wood DG, Brown MB, Jones SA. Understanding heat facilitated drug transport across human epidermis. Eur J Pharm Biopharm. 2012. https://doi.org/10.1016/j.ejpb.2012.03.019.
Farahmand S, Maibach HI. Transdermal drug pharmacokinetics in man: Interindividual variability and partial prediction. Int J Pharm. 2009. https://doi.org/10.1016/j.ijpharm.2008.11.020.
Rougier A, Lotte C, Corcuff P, Maibach HI. Relationship between skin permeability and corneocyte size according to anatomic site, age, and sex in man. J Soc Cosmet Chem. 1988.
Darlenski R, Fluhr JW. Influence of skin type, race, sex, and anatomic location on epidermal barrier function. Clin Dermatol. 2012. https://doi.org/10.1016/j.clindermatol.2011.08.013.
Singh I, Morris AP. Performance of transdermal therapeutic systems: effects of biological factors. Int J Pharm Investig. 2011. https://doi.org/10.4103/2230-973X.76721.
Ashburn MA, Ogden LL, Zhang J, Love G, Basta SV. The pharmacokinetics of transdermal fentanyl delivered with and without controlled heat. J Pain. 2003. https://doi.org/10.1016/s1526-5900(03)00618-7.
Marriott TB, Charney MR, Stanworth S. Effects of application durations and heat on the pharmacokinetic properties of drug delivered by a lidocaine/tetracaine patch: a randomized, open-label, controlled study in healthy volunteers. Clin Ther. 2012. https://doi.org/10.1016/j.clinthera.2012.08.008.
Moore KT, Sathyan G, Richarz U, Natarajan J, Vandenbossche J. Randomized 5-treatment crossover study to assess the effects of external heat on serum fentanyl concentrations during treatment with transdermal fentanyl systems. J Clin Pharmacol. 2012. https://doi.org/10.1177/0091270011411710.
Petersen KK, Rousing ML, Jensen C, Arendt-Nielsen L, Gazerani P. Effect of local controlled heat on transdermal delivery of nicotine. Int J Physiol Pathophysiol Pharmacol. 2011.
Prodduturi S, Sadrieh N, Wokovich AM, Doub WH, Westenberger BJ, Buhse L. Transdermal delivery of fentanyl from matrix and reservoir systems: effect of heat and compromised skin. J Pharm Sci. 2010. https://doi.org/10.1002/jps.22004.
Otto DP, de Villiers MM. The experimental evaluation and molecular dynamics simulation of a heat-enhanced transdermal delivery system. AAPS PharmSciTech. 2013. https://doi.org/10.1208/s12249-012-9900-6.
Vanakoski J, Seppala T, Sievi E, Lunell E. Exposure to high ambient temperature increases absorption and plasma concentrations of transdermal nicotine. Clin Pharmacol Ther. 1996. https://doi.org/10.1016/S0009-9236(96)90057-0.
Treffel P, Muret P, Muret-D'Aniello P, Coumes-Marquet S, Agache P. Effect of occlusion on in vitro percutaneous absorption of two compounds with different physicochemical properties. Skin Pharmacol. 1992. https://doi.org/10.1159/000211027.
Acknowledgements
We are grateful for the efforts of Jennifer Marron and other personnel at the General Clinical Research Center at the University of Maryland as well as Danielle Fox for her endless guidance on clinical study management. Recruitment for the study included/was done via ResearchMatch, a national health volunteer registry that was created by several academic institutions and supported by the U.S. National Institutes of Health as part of the Clinical Translational Science Award (CTSA) program. ResearchMatch has a large population of volunteers who have consented to be contacted by researchers about health studies for which they may be eligible. Review and approval for this study and all procedures was obtained from the University of Maryland, Baltimore Institutional Review Board. Biospecimens were provided by the NCI funded Cooperative Human Tissue Network (CHTN). Other investigators may have received specimens from the same tissue specimens. The CHTN is comprised of six academic institutions who collect and distribute remnant human biospecimens from routine surgical and autopsy procedures to investigators for basic and applied science to advance biomedical research.
Funding
Funding for this project was made possible, in part, by the University of Maryland Baltimore, School of Pharmacy Mass Spectrometry Center (Research Award SOP1841-IQB2014), the University of Maryland Baltimore, Institute for Clinical & Translational Research (ICTR) voucher program, and the University of Maryland Baltimore Pharmaceutical Sciences Department Fellowship and Merit Awards.
Author information
Authors and Affiliations
Contributions
Paige Zambrana: writing–original draft; data curation; formal analysis; investigation; methodology; validation; visualization. Dana C. Hammell: writing–review and editing; data curation; project administration; resources. Audra L. Stinchcomb: conceptualization; funding acquisition; methodology; supervision; writing–review and editing
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The University of Maryland, Baltimore Investigational Review Board provided approval for protocol and informed consents for subjects in accordance with the Code of Federal Regulations and local requirements. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Consent for publication
The publisher has the authors’ permission to publish this contribution.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zambrana, P.N., Hammell, D.C. & Stinchcomb, A.L. Advanced harmonization techniques result in accurate establishment of in vitro–in vivo correlations for oxybenzone from four complex dermal formulations with reapplication. Drug Deliv. and Transl. Res. 13, 275–291 (2023). https://doi.org/10.1007/s13346-022-01186-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01186-7