Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a serious and often persistent adverse consequence of certain chemotherapeutic agents. It is a major dose-limiting factor of many first-line chemotherapies, affecting 20–50% of patients at standard doses and nearly all patients at high doses. As cancer survivorship continues to increase with improvements in early diagnosis and treatment, more patients will experience CIPN despite completing cancer treatment, which interferes with recovery, leading to chronic pain and worsening quality of life. The National Cancer Institute has identified CIPN as a priority in translational research. To date, there are no FDA-approved drugs for preventing or treating CIPN, with emerging debate on mechanisms and promising new targets. This review highlights current literature and suggests novel approaches to CIPN based on proposed mechanisms of action that aim either to confer neuroprotection against chemotherapy-induced neurotoxicity or reverse the downstream effects of painful neuropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
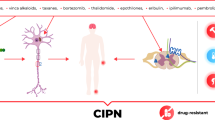
Chemotherapy-induced peripheral neuropathy (CIPN) is a common and debilitating complication of several anti-neoplastic agents, including taxanes (paclitaxel, docetaxel), platinum derivatives (carboplatin, cisplatin, oxaliplatin), vinca alkaloids (vincristine, vinblastine), and proteasome inhibitors (bortezomib) [1, 2]. Taxanes and vinca alkaloids belong to a class of microtubule inhibitors [3]. Taxanes stabilize microtubules, so they cannot depolymerize and function properly, leading to cell cycle arrest, whereas vinca alkaloids inhibit β-tubulin polymerization, preventing mitotic spindle formation, leading to mitotic arrest and cell death by apoptosis [3]. A recent study demonstrated that integrins, cell surface receptors that mediate cell-extracellular matrix interaction, protect against paclitaxel-induced neuropathy. Paclitaxel exposure altered the branching pattern of nociceptive neurons in Drosophila — integrin overexpression rescued compromised interaction between sensory neurons and the extracellular matrix and restored lost nocifensive escape behavior to thermal noxious stimuli [4].
Platinum-based chemotherapies, or “alkylating-like agents” due to their similarity in mechanism to classical alkylating agents, cross-link DNA and form interstrand DNA-platinum adducts, leading to non-specific cell cycle arrest [5]. Bortezomib, a proteasome inhibitor and first-line treatment for multiple myeloma, inhibits the proteasome-ubiquitination pathway, preventing degradation of pro-apoptotic proteins [5, 6]. At clinically relevant doses, bortezomib alters microtubule stabilization by increasing tubulin polymerization, disrupting axonal transport of mitochondria, and promoting cytotoxicity [7]. In line with the sensory axonopathy associated with bortezomib treatment, multiple studies have shown that bortezomib significantly increases polymerized α-tubulin in the sciatic nerve and dorsal root ganglia of rats [8, 9]. A recent study highlighted the role of delta 2 tubulin, a marker of hyperstable microtubules, in bortezomib-induced axonopathy/loss of axonal transport of mitochondria and found D2 accumulation to be sufficient and necessary in driving this process [10]. For these anti-neoplastic agents, especially vincristine which has the greatest affinity for tubulin among vinca alkaloids, neurotoxicity is a major dose-limiting complication.
While CIPN pathophysiology is complex and should not be extrapolated from other peripheral neuropathies (e.g., diabetic neuropathy), signs and symptoms typically manifest in a “glove and stocking” anatomical distribution, simultaneously affecting hands and feet bilaterally, with distal-to-proximal symptom progression [11]. CIPN most commonly presents through sensory changes but motor and autonomic deficits can ensue. Signs and symptoms include evoked or spontaneous pain that ranges from tingling (“pins and needles” sensation) to stabbing or burning pain. Mechanical and cold allodynia (pain from pressure and cold temperature, otherwise innocuous), numbness, and weakness are common characteristics of CIPN [12].
CIPN is sometimes mild and reversible, whereas in other cases, it can be severe and irreversible, interfering with daily activities. CIPN prevalence depends on the chemotherapeutic agent, dosage, and duration. In one meta-analysis, it was found that 68.1% of patients experience CIPN within the first month after chemotherapy, 60.0% at 3 months, and 30.0% at 6 months and beyond (considered chronic CIPN) [13]. However, given the heterogeneity of CIPN risk with no validated clinical biomarkers, it is important to identify risk and protective factors for CIPN to better predict outcomes, understand its etiology and underlying mechanisms, and develop a personalized approach to CIPN prevention and treatment.
Clinical risk factors for develo** CIPN include a history of pre-existing neuropathy, comorbidities such as diabetes mellitus, lifestyle factors like smoking, and decreased creatinine clearance [13]. Interestingly, a history of autoimmune disease was found to be associated with reduced risk of CIPN [14]. Predisposing genetic factors include single nucleotide polymorphisms (SNPs) in FGD4, a gene associated with hereditary peripheral neuropathy in Charcot-Marie-Tooth disease, and genes involved in dysfunctional receptor activity resulting in neuronal apoptosis and prolonged muscle contraction in patients with CIPN treated with platinum drugs for breast and colon cancer, respectively [15, 16]. These CIPN-associated genetic markers may partly explain common symptoms that patients experience, including altered sensation due to apoptosis in dorsal root ganglion (DRG) sensory neurons and muscle ataxia. Cumulative dosing and infusion timing of the chemotherapeutic agent and SNPs in genes coding for voltage-gated sodium channels and myelinating Schwann cell-associated proteins are additional contributing risk factors related to CIPN mechanisms [17].
Status of Treatments for CIPN
The American Society of Clinical Oncology (ASCO) recently updated their guidelines on CIPN preventive and treatment practices [18]. Several agents that have been investigated lack evidence to support their use as potential therapies. Acetyl-L-carnitine was strongly discouraged for prevention of CIPN. Other “natural” approaches such as all trans retinoic acid (vitamin A metabolite) and antioxidants like vitamin E and glutathione, omega-3 fatty acids, and calcium magnesium infusions were not recommended as no benefits for CIPN prevention were found. Calmangafodipir, an intravenous contrast agent for magnetic resonance imaging, explored for its superoxide dismutase-like (anti-oxidant) activity, was not recommended [19]. Other chemoprotectants such as nimodipine, a calcium channel blocker, and amifostine, a cytoprotective agent against cisplatin-induced nephrotoxicity, have shown mixed results and are currently not recommended for CIPN [20, 21]. RhuLIF (human recombinant leukemia-inhibiting factor), a member of the cytokine family that includes IL-6, proposed to be neuroprotective against peripheral neuropathy, was ineffective against CIPN in a randomized, double-blind, placebo-controlled phase II clinical trial [22].
Established neuropathic pain treatments include tricyclic antidepressants (TCAs), dual serotonin and norepinephrine reuptake inhibitors (SNRIs), anticonvulsants, and opioid agonists [23]. Of these, only the SNRI duloxetine, which is US Food and Drug Administration (FDA)-approved for treating major depressive disorder and diabetic neuropathy, exhibits moderate efficacy in treating CIPN and is often used clinically at doses from 60 to 120 mg/day [24]. No other intervention in this drug class has shown comparable therapeutic effects with a favorable risk/benefit ratio. The TCA nortriptyline has a good safety profile but did not significantly relieve paresthesia or pain in a phase III randomized, double-blind, crossover trial [25, 26]. However, one clinical advantage to using antidepressants is improvement in mood which can help with overall treatment. Anticonvulsants like gabapentin and pregabalin that block voltage-gated calcium channels and decrease excitatory neurotransmission have conflicting efficacy data, and side effects including somnolence and dizziness. Opioids have many adverse side effects with chronic use and are not considered first-line treatment for neuropathic pain.
In general, a safer and more effective therapeutic approach may involve combination therapy. For example, the combination of morphine and gabapentin reduced neuropathic pain significantly more than either agent alone in a randomized controlled trial [27]. Combination therapy with nortriptyline and gabapentin led to a synergistic effect [28]. More clinical trials investigating combination therapy specific for CIPN are needed. A compounded topical analgesic gel consisting of baclofen (γ-amino-butyric acid [GABA]-B receptor agonist), amitriptyline (TCA), and ketamine (N-methyl-D-aspartate [NMDA] receptor antagonist) has shown mild benefit in treating CIPN symptoms with no signs of systemic toxicity; however, existing data are inconclusive and further research is required [29].
Drug-repositioning studies can help identify new or secondary actions of already-approved drugs, which may prove more efficient than de novo drug development [30]. Many drug candidates for CIPN prevention and treatment can be re-purposed based on their mechanism (e.g., neuronal damage) or by screening chemical libraries to test drugs with unclear actions to identify the mechanism, while also investigating the safety profile of these drugs to prevent further CIPN progression [30].
Proposed Mechanisms of CIPN
Mechanisms by which chemotherapy-induced neurotoxicity translates to CIPN are complex and multi-factorial. Suggested mechanisms include transporter-mediated uptake of chemotherapy drug, oxidative stress secondary to mitochondrial damage, microtubule disruption and subsequent loss of axonal transport, axonal degeneration, damage to DRG sensory neurons, abnormal discharge of pain fibers (A \(\delta\) and C fibers), upregulation of proinflammatory cytokines, changes to ion conductance, and inhibition of growth factors [31, 133]. Finally, we note that because of our limited understanding of CIPN pathophysiology and its heterogenous presentation, early patient education and discussion with clinicians is needed to alleviate the burden of CIPN and improve quality of life in cancer patients and survivors.
References
Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9-17. https://doi.org/10.1007/pl00007853
Swain SM, Arezzo JC. Neuropathy associated with microtubule inhibitors: diagnosis, incidence, and management. Clin Adv Hematol Oncol. 2008;6(6):455-467
Perez EA. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther. 2009;8(8):2086-2095. https://doi.org/10.1158/1535-7163.MCT-09-0366
Shin GJ, Pero ME, Hammond LA, et al. Integrins protect sensory neurons in models of paclitaxel-induced peripheral sensory neuropathy. PNAS. 2021;118(15). https://doi.org/10.1073/pnas.2006050118
Fukuda Y, Li Y, Segal RA. A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Front Neurosci. 2017;11:481. https://doi.org/10.3389/fnins.2017.00481
Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82(1):51-77. https://doi.org/10.1016/j.critrevonc.2011.04.012
Staff NP, Podratz JL, Grassner L, et al. Bortezomib alters microtubule polymerization and axonal transport in rat dorsal root ganglion neurons. Neurotoxicology. 2013;39:124-131. https://doi.org/10.1016/j.neuro.2013.09.001
Meregalli C, Chiorazzi A, Carozzi VA, et al. Evaluation of tubulin polymerization and chronic inhibition of proteasome as cytotoxicity mechanisms in bortezomib-induced peripheral neuropathy. Cell Cycle. 2014;13(4):612-621. https://doi.org/10.4161/cc.27476
Poruchynsky MS, Sackett DL, Robey RW, Ward Y, Annunziata C, Fojo T. Proteasome inhibitors increase tubulin polymerization and stabilization in tissue culture cells: a possible mechanism contributing to peripheral neuropathy and cellular toxicity following proteasome inhibition. Cell Cycle. 2008;7(7):940-949. https://doi.org/10.4161/cc.7.7.5625
Pero ME, Meregalli C, Qu X, et al. Pathogenic role of delta 2 tubulin in bortezomib-induced peripheral neuropathy. Proc Natl Acad Sci U S A. 2021;118(4):e2012685118. https://doi.org/10.1073/pnas.2012685118
Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. 2019;20(6). https://doi.org/10.3390/ijms20061451
Han Y, Smith MTP. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front Pharmacol. 2013;4. https://doi.org/10.3389/fphar.2013.00156
Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. PAIN. 2014;155(12):2461-2470. https://doi.org/10.1016/j.pain.2014.09.020
Hershman DL, Till C, Wright JD, et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group Clinical Trials. J Clin Oncol. 2016;34(25):3014-3022. https://doi.org/10.1200/JCO.2015.66.2346
Baldwin RM, Owzar K, Zembutsu H, et al. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res. 2012;18(18):5099-5109. https://doi.org/10.1158/1078-0432.CCR-12-1590
Won H-H, Lee J, Park JO, et al. Polymorphic markers associated with severe oxaliplatin-induced, chronic peripheral neuropathy in colon cancer patients. Cancer. 2012;118(11):2828-2836. https://doi.org/10.1002/cncr.26614
Boyette-Davis JA, Walters ET, Dougherty PM. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag. 2015;5(4):285-296. https://doi.org/10.2217/pmt.15.19
Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. JCO. 2020;38(28):3325-3348. https://doi.org/10.1200/JCO.20.01399
Karlsson JOG, Ignarro LJ, Lundström I, Jynge P, Almén T. Calmangafodipir [Ca4Mn(DPDP)5], mangafodipir (MnDPDP) and MnPLED with special reference to their SOD mimetic and therapeutic properties. Drug Discov Today. 2015;20(4):411-421. https://doi.org/10.1016/j.drudis.2014.11.008
Cassidy J, Paul J, Soukop M, et al. Clinical trials of nimodipine as a potential neuroprotector in ovarian cancer patients treated with cisplatin. Cancer Chemother Pharmacol. 1997;41(2):161-166. https://doi.org/10.1007/s002800050723
Sastry J, Kellie SJ. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr Hematol Oncol. 2005;22(5):441-445. https://doi.org/10.1080/08880010590964381
Davis ID, Kiers L, MacGregor L, et al. A randomized, double-blinded, placebo-controlled phase II trial of recombinant human leukemia inhibitory factor (rhuLIF, emfilermin, AM424) to prevent chemotherapy-induced peripheral neuropathy. Clin Cancer Res. 2005;11(5):1890-1898. https://doi.org/10.1158/1078-0432.CCR-04-1655
Ibrahim EY, Ehrlich BE. Prevention of chemotherapy-induced peripheral neuropathy: a review of recent findings. Crit Rev Oncol Hematol. 2020;145:102831. https://doi.org/10.1016/j.critrevonc.2019.102831
Smith EML, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359-1367. https://doi.org/10.1001/jama.2013.2813
Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy. Cancer. 2007;110(9):2110-2118. https://doi.org/10.1002/cncr.23008
Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. PAIN. 2002;98(1):195-203. https://doi.org/10.1016/S0304-3959(02)00047-7
Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352(13):1324-1334. https://doi.org/10.1056/NEJMoa042580
Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. The Lancet. 2009;374(9697):1252-1261. https://doi.org/10.1016/S0140-6736(09)61081-3
Barton DL, Wos EJ, Qin R, et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer. 2011;19(6):833-841. https://doi.org/10.1007/s00520-010-0911-0
Yamamoto S, Egashira N. Drug repositioning for the prevention and treatment of chemotherapy-induced peripheral neuropathy: a mechanism- and screening-based strategy. Front Pharmacol. 2021;11. https://doi.org/10.3389/fphar.2020.607780
Areti A, Yerra VG, Naidu V, Kumar A. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014;2:289-295. https://doi.org/10.1016/j.redox.2014.01.006
**ao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-l-carnitine. Pain. 2008;135(3):262-270. https://doi.org/10.1016/j.pain.2007.06.001
Sałat K. Chemotherapy-induced peripheral neuropathy: part 1—current state of knowledge and perspectives for pharmacotherapy. Pharmacol Rep. 2020;72(3):486-507. https://doi.org/10.1007/s43440-020-00109-y
Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Phil Trans Royal Soc London Series I. 1850;140:423-429
Di Cesare Mannelli L, Pacini A, Bonaccini L, Zanardelli M, Mello T, Ghelardini C. Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J Pain. 2013;14(12):1585-1600. https://doi.org/10.1016/j.jpain.2013.08.002
Huang Z-Z, Li D, Ou-Yang H-D, et al. Cerebrospinal fluid oxaliplatin contributes to the acute pain induced by systemic administration of oxaliplatin. Anesthesiology. 2016;124(5):1109-1121. https://doi.org/10.1097/ALN.0000000000001084
Coleman MP, Höke A. Programmed axon degeneration: from mouse to mechanism to medicine. Nat Rev Neurosci. 2020;21(4):183-196. https://doi.org/10.1038/s41583-020-0269-3
Osterloh JM, Yang J, Rooney TM, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337(6093):481-484. https://doi.org/10.1126/science.1223899
Bosanac T, Hughes RO, Engber T, et al. Pharmacological SARM1 inhibition protects axon structure and function in paclitaxel-induced peripheral neuropathy. Brain. Published online May 8 2021:awab184. https://doi.org/10.1093/brain/awab184
Cetinkaya-Fisgin A, Luan X, Reed N, Jeong YE, Oh BC, Hoke A. Cisplatin induced neurotoxicity is mediated by Sarm1 and calpain activation. Sci Rep. 2020;10(1):21889. https://doi.org/10.1038/s41598-020-78896-w
Gilley J, Mayer PR, Yu G, Coleman MP. Low levels of NMNAT2 compromise axon development and survival. Hum Mol Genet. 2019;28(3):448-458. https://doi.org/10.1093/hmg/ddy356
Siau C, Bennett GJ. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg. 2006;102(5):1485-1490. https://doi.org/10.1213/01.ane.0000204318.35194.ed
Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/Bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005;65(9):3828-3836. https://doi.org/10.1158/0008-5472.CAN-04-3684
Khanna R, Yu J, Yang X, et al. Targeting the CaVα–CaVβ interaction yields an antagonist of the N-type CaV2.2 channel with broad antinociceptive efficacy. PAIN. 2019;160(7):1644–1661. https://doi.org/10.1097/j.pain.0000000000001524
Flatters SJL, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. PAIN. 2004;109(1):150-161. https://doi.org/10.1016/j.pain.2004.01.029
Kerckhove N, Pereira B, Soriot-Thomas S, et al. Efficacy and safety of a T-type calcium channel blocker in patients with neuropathic pain: a proof-of-concept, randomized, double-blind and controlled trial. Eur J Pain. 2018;22(7):1321-1330. https://doi.org/10.1002/ejp.1221
Cai S, Tuohy P, Ma C, et al. A modulator of the low-voltage-activated T-type calcium channel that reverses HIV glycoprotein 120-, paclitaxel-, and spinal nerve ligation-induced peripheral neuropathies. Pain. 2020;161(11):2551-2570. https://doi.org/10.1097/j.pain.0000000000001955
Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nature Rev Drug Discov. 2007;6(8):662-680. https://doi.org/10.1038/nrd2222
Janes K, Esposito E, Doyle T, et al. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. PAIN. 2014;155(12):2560-2567. https://doi.org/10.1016/j.pain.2014.09.016
Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2(4):236-240. https://doi.org/10.4103/2231-4040.90879
Quintão NLM, Santin JR, Stoeberl LC, Corrêa TP, Melato J, Costa R. Pharmacological treatment of chemotherapy-induced neuropathic pain: PPARγ agonists as a promising tool. Front Neurosci. 2019;13. https://doi.org/10.3389/fnins.2019.00907
Khasabova IA, Khasabov SG, Olson JK, et al. Pioglitazone, a PPARγ agonist, reduces cisplatin-evoked neuropathic pain by protecting against oxidative stress. Pain. 2019;160(3):688-701. https://doi.org/10.1097/j.pain.0000000000001448
Zanardelli M, Micheli L, Cinci L, Failli P, Ghelardini C, Di Cesare Mannelli L. Oxaliplatin neurotoxicity involves peroxisome alterations. PPARγ agonism as preventive pharmacological approach. PLoS One. 2014;9(7). https://doi.org/10.1371/journal.pone.0102758
Kelley MR, Wikel JH, Guo C, et al. Identification and characterization of new chemical entities targeting apurinic/apyrimidinic endonuclease 1 for the prevention of chemotherapy-induced peripheral neuropathy. J Pharmacol Exp Ther. 2016;359(2):300-309. https://doi.org/10.1124/jpet.116.235283
Mannelli LDC, Zanardelli M, Failli P, Ghelardini C. Oxaliplatin-induced neuropathy: oxidative stress as pathological mechanism Protective effect of silibinin. J Pain. 2012;13(3):276-284. https://doi.org/10.1016/j.jpain.2011.11.009
Fumagalli G, Monza L, Cavaletti G, Rigolio R, Meregalli C. Neuroinflammatory process involved in different preclinical models of chemotherapy-induced peripheral neuropathy. Front Immunol. 2021;11. https://doi.org/10.3389/fimmu.2020.626687
Wang X-M, Lehky TJ. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59(1):3-9. https://doi.org/10.1016/j.cyto.2012.03.027
Cata JP, Weng H-R, Chen J-H, Dougherty PM. Altered discharges of spinal wide dynamic range neurons and down-regulation of glutamate transporter expression in rats with paclitaxel-induced hyperalgesia. Neuroscience. 2006;138(1):329-338. https://doi.org/10.1016/j.neuroscience.2005.11.009
Brandolini L, Castelli V, Aramini A, et al. DF2726A, a new IL-8 signalling inhibitor, is able to counteract chemotherapy-induced neuropathic pain. Sci Rep. 2019;9(1):11729. https://doi.org/10.1038/s41598-019-48231-z
Brandolini L, Cristiano L, Fidoamore A, et al. Targeting CXCR1 on breast cancer stem cells: signaling pathways and clinical application modelling. Oncotarget. 2015;6(41):43375-43394. https://doi.org/10.18632/oncotarget.6234
Krukowski K, Eijkelkamp N, Laumet G, et al. CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. Journal of Neuroscience. 2016;36(43):11074-11083. https://doi.org/10.1523/JNEUROSCI.3708-15.2016
Honore P, Donnelly-Roberts D, Namovic MT, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319(3):1376-1385. https://doi.org/10.1124/jpet.106.111559
Melemedjian OK, Asiedu MN, Tillu DV, et al. Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain. Mol Pain. 2011;7:70. https://doi.org/10.1186/1744-8069-7-70
Inyang KE, McDougal TA, Ramirez ED, et al. Alleviation of paclitaxel-induced mechanical hypersensitivity and hyperalgesic priming with AMPK activators in male and female mice. Neurobiol Pain. 2019;6:100037. https://doi.org/10.1016/j.ynpai.2019.100037
Mao-Ying Q-L, Kavelaars A, Krukowski K, et al. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLOS One. 2014;9(6):e100701. https://doi.org/10.1371/journal.pone.0100701
Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247-257. https://doi.org/10.1002/emmm.201000080
Bessaguet F, Danigo A, Bouchenaki H, et al. Neuroprotective effect of angiotensin II type 2 receptor stimulation in vincristine-induced mechanical allodynia. Pain. 2018;159(12):2538-2546. https://doi.org/10.1097/j.pain.0000000000001361
Fujita T, Hirooka K, Nakamura T, et al. Neuroprotective effects of angiotensin II type 1 receptor (AT1-R) blocker via modulating AT1-R signaling and decreased extracellular glutamate levels. Invest Ophthalmol Vis Sci. 2012;53(7):4099-4110. https://doi.org/10.1167/iovs.11-9167
Kim E, Hwang S-H, Kim H-K, Abdi S, Kim HK. Losartan, an angiotensin II type 1 receptor antagonist, alleviates mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain by inhibiting inflammatory cytokines in the dorsal root ganglia. Mol Neurobiol. 2019;56(11):7408-7419. https://doi.org/10.1007/s12035-019-1616-0
Maiarù M, Morgan OB, Mao T, et al. The stress regulator FKBP51: a novel and promising druggable target for the treatment of persistent pain states across sexes. Pain. 2018;159(7):1224-1234. https://doi.org/10.1097/j.pain.0000000000001204
Bruna J, Videla S, Argyriou AA, et al. Efficacy of a novel sigma-1 receptor antagonist for oxaliplatin-induced neuropathy: a randomized, double-blind, placebo-controlled phase IIa clinical trial. Neurotherapeutics. 2018;15(1):178-189. https://doi.org/10.1007/s13311-017-0572-5
Krukowski K, Ma J, Golonzhka O, et al. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain. 2017;158(6):1126-1137. https://doi.org/10.1097/j.pain.0000000000000893
Liu H, Smith CB, Schmidt MS, et al. Pharmacological bypass of NAD+ salvage pathway protects neurons from chemotherapy-induced degeneration. Proc Natl Acad Sci U S A. 2018;115(42):10654-10659
Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165(5):1260-1287. https://doi.org/10.1111/j.1476-5381.2011.01724.x
Leblanc AF, Sprowl JA, Alberti P, et al. OATP1B2 deficiency protects against paclitaxel-induced neurotoxicity. J Clin Invest. 128(2):816–825. https://doi.org/10.1172/JCI96160
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04205903 (2019)
Vanderah TW. Pathophysiology of pain. Med Clin North Am. 2007;91(1):1-12. https://doi.org/10.1016/j.mcna.2006.10.006
Boyette-Davis JA, Hou S, Abdi S, Dougherty PM. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Management. 2018;8(5):363-375. https://doi.org/10.2217/pmt-2018-0020
Tatsushima Y, Egashira N, Kawashiri T, et al. Involvement of substance P in peripheral neuropathy induced by paclitaxel but not oxaliplatin. J Pharmacol Exp Ther. 2011;337(1):226-235. https://doi.org/10.1124/jpet.110.175976
Dick IE, Brochu RM, Purohit Y, Kaczorowski GJ, Martin WJ, Priest BT. Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. J Pain. 2007;8(4):315-324. https://doi.org/10.1016/j.jpain.2006.10.001
Rossignol J, Cozzi B, Liebaert F, et al. High concentration of topical amitriptyline for treating chemotherapy-induced neuropathies. Support Care Cancer. 2019;27(8):3053-3059. https://doi.org/10.1007/s00520-018-4618-y
Yin M, Kim Y-O, Choi J-I, et al. Antinociceptive role of neurotensin receptor 1 in rats with chemotherapy-induced peripheral neuropathy. Korean J Pain. 2020;33(4):318-325. https://doi.org/10.3344/kjp.2020.33.4.318
Christou N, Blondy S, David V, et al. Neurotensin pathway in digestive cancers and clinical applications: an overview. Cell Death Dis. 2020;11(12):1027. https://doi.org/10.1038/s41419-020-03245-8
Thibault K, Van Steenwinckel J, Brisorgueil M-J, et al. Serotonin 5-HT2A receptor involvement and Fos expression at the spinal level in vincristine-induced neuropathy in the rat. PAIN. 2008;140(2):305-322. https://doi.org/10.1016/j.pain.2008.09.006
Osikowicz M, Mika J, Przewlocka B. The glutamatergic system as a target for neuropathic pain relief. Exp Physiol. 2013;98(2):372-384. https://doi.org/10.1113/expphysiol.2012.069922
Carozzi VA, Chiorazzi A, Canta A, et al. Glutamate carboxypeptidase inhibition reduces the severity of chemotherapy-induced peripheral neurotoxicity in rat. Neurotox Res. 2010;17(4):380-391. https://doi.org/10.1007/s12640-009-9114-1
Ghelardini C, Menicacci C, Cerretani D, Bianchi E. Spinal administration of mGluR5 antagonist prevents the onset of bortezomib induced neuropathic pain in rat. Neuropharmacology. 2014;86:294-300. https://doi.org/10.1016/j.neuropharm.2014.08.004
Weng H-R, Aravindan N, Cata JP, Chen J-H, Shaw ADS, Dougherty PM. Spinal glial glutamate transporters downregulate in rats with taxol-induced hyperalgesia. Neurosci Lett. 2005;386(1):18-22. https://doi.org/10.1016/j.neulet.2005.05.049
Sałat K, Furgała A, Sałat R. Interventional and preventive effects of aripiprazole and ceftriaxone used alone or in combination on oxaliplatin-induced tactile and cold allodynia in mice. Biomed Pharmacother. 2019;111:882-890. https://doi.org/10.1016/j.biopha.2019.01.008
Park BY, Park SH, Kim WM, Yoon MH, Lee HG. Antinociceptive effect of memantine and morphine on vincristine-induced peripheral neuropathy in rats. Korean J Pain. 2010;23(3):179-185. https://doi.org/10.3344/kjp.2010.23.3.179
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03709888 (2018)
Yamamoto S, Ushio S, Egashira N, et al. Excessive spinal glutamate transmission is involved in oxaliplatin-induced mechanical allodynia: a possibility for riluzole as a prophylactic drug. Sci Rep. 2017;7(1):9661. https://doi.org/10.1038/s41598-017-08891-1
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03722680 (2018)
Bravo D, Ibarra P, Retamal J, et al. Pannexin 1: A novel participant in neuropathic pain signaling in the rat spinal cord. PAIN. 2014;155(10):2108-2115. https://doi.org/10.1016/j.pain.2014.07.024
Di Cesare Mannelli L, Marcoli M, Micheli L, et al. Oxaliplatin evokes P2X7-dependent glutamate release in the cerebral cortex: a pain mechanism mediated by Pannexin 1. Neuropharmacology. 2015;97:133-141. https://doi.org/10.1016/j.neuropharm.2015.05.037
Masocha W. Targeting the endocannabinoid system for prevention or treatment of chemotherapy-induced neuropathic pain: studies in animal models. Pain Res Manag. 2018;2018:5234943. https://doi.org/10.1155/2018/5234943
O’Hearn S, Diaz P, Wan BA, et al. Modulating the endocannabinoid pathway as treatment for peripheral neuropathic pain: a selected review of preclinical studies. Ann Palliat Med. 2017;6(Suppl 2):S209-S214. https://doi.org/10.21037/apm.2017.08.04
Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3364-3378. https://doi.org/10.1098/rstb.2011.0389
Mijangos-Moreno S, Poot-Aké A, Arankowsky-Sandoval G, Murillo-Rodríguez E. Intrahypothalamic injection of cannabidiol increases the extracellular levels of adenosine in nucleus accumbens in rats. Neurosci Res. 2014;84:60-63. https://doi.org/10.1016/j.neures.2014.04.006
Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2006;372(5):354-361. https://doi.org/10.1007/s00210-006-0033-x
Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134(4):845-852. https://doi.org/10.1038/sj.bjp.0704327
Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037-1043. https://doi.org/10.1007/s11064-005-6978-1
Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol. 2014;171(3):636-645. https://doi.org/10.1111/bph.12439
Brenneman DE, Kinney WA, Ward SJ. Knockdown siRNA Targeting the mitochondrial sodium-calcium exchanger-1 inhibits the protective effects of two cannabinoids against acute paclitaxel toxicity. J Mol Neurosci. 2019;68(4):603-619. https://doi.org/10.1007/s12031-019-01321-z
Blanton HL, Brelsfoard J, DeTurk N, et al. Cannabinoids: current and future options to treat chronic and chemotherapy-induced neuropathic pain. Drugs. 2019;79(9):969-995. https://doi.org/10.1007/s40265-019-01132-x
Uhelski ML, Khasabova IA, Simone DA. Inhibition of anandamide hydrolysis attenuates nociceptor sensitization in a murine model of chemotherapy-induced peripheral neuropathy. J Neurophysiol. 2015;113(5):1501-1510. https://doi.org/10.1152/jn.00692.2014
Sierra S, Gupta A, Gomes I, et al. Targeting cannabinoid 1 and delta opioid receptor heteromers alleviates chemotherapy-induced neuropathic pain. ACS Pharmacol Transl Sci. 2019;2(4):219-229. https://doi.org/10.1021/acsptsci.9b00008
Curry ZA, Wilkerson JL, Bagdas D, et al. Monoacylglycerol lipase inhibitors reverse paclitaxel-induced nociceptive behavior and proinflammatory markers in a mouse model of chemotherapy-induced neuropathy. J Pharmacol Exp Ther. 2018;366(1):169-183. https://doi.org/10.1124/jpet.117.245704
Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol Res. 2013;67(1):94-109. https://doi.org/10.1016/j.phrs.2012.10.013
Malan TP, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol. 2003;3(1):62-67. https://doi.org/10.1016/s1471-4892(02)00004-8
Naguib M, Xu JJ, Diaz P, et al. Prevention of paclitaxel-induced neuropathy through activation of the central cannabinoid type 2 receptor system. Anesth Analg. 2012;114(5):1104-1120. https://doi.org/10.1213/ANE.0b013e31824b0191
Carrasco C, Naziroǧlu M, Rodríguez AB, Pariente JA. Neuropathic pain: delving into the oxidative origin and the possible implication of transient receptor potential channels. Front Physiol. 2018;9. https://doi.org/10.3389/fphys.2018.00095
Nazıroğlu M, Braidy N. Thermo-sensitive TRP channels: novel targets for treating chemotherapy-induced peripheral pain. Front Physiol. 2017;8:1040. https://doi.org/10.3389/fphys.2017.01040
Alessandri-Haber N. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neuroscience. 2004;24(18):4444-4452. https://doi.org/10.1523/JNEUROSCI.0242-04.2004
Trevisan G, Materazzi S, Fusi C, et al. Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. 2013;73(10):3120-3131. https://doi.org/10.1158/0008-5472.CAN-12-4370
Fattori V, Hohmann MSN, Rossaneis AC, Pinho-Ribeiro FA, Verri WA. Capsaicin: current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules. 2016;21(7). https://doi.org/10.3390/molecules21070844
Anand P, Elsafa E, Privitera R, et al. Rational treatment of chemotherapy-induced peripheral neuropathy with capsaicin 8% patch: from pain relief towards disease modification. J Pain Res. 2019;12:2039-2052. https://doi.org/10.2147/JPR.S213912
Fallon MT, Storey DJ, Krishan A, et al. Cancer treatment-related neuropathic pain: proof of concept study with menthol—a TRPM8 agonist. Support Care Cancer. 2015;23(9):2769-2777. https://doi.org/10.1007/s00520-015-2642-8
Romero HK, Christensen SB, Di Cesare Mannelli L, et al. Inhibition of α9α10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc Natl Acad Sci U S A. 2017;114(10):E1825-E1832
Di Cesare Mannelli L, Pacini A, Matera C, et al. Involvement of α7 nAChR subtype in rat oxaliplatin-induced neuropathy: effects of selective activation. Neuropharmacology. 2014;79:37-48. https://doi.org/10.1016/j.neuropharm.2013.10.034
Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain. 2007;131(3):243-257. https://doi.org/10.1016/j.pain.2007.07.026
Angus M, Ruben P. Voltage gated sodium channels in cancer and their potential mechanisms of action. Channels (Austin). 2019;13(1):400-409. https://doi.org/10.1080/19336950.2019.1666455
House CD, Wang B-D, Ceniccola K, et al. Voltage-gated Na+ channel activity increases colon cancer transcriptional activity and invasion via persistent MAPK signaling. Sci Rep. 2015;5(1):11541. https://doi.org/10.1038/srep11541
Argyriou AA, Cavaletti G, Antonacopoulou A, et al. Voltage-gated sodium channel polymorphisms play a pivotal role in the development of oxaliplatin-induced peripheral neurotoxicity: results from a prospective multicenter study. Cancer. 2013;119(19):3570-3577. https://doi.org/10.1002/cncr.28234
Alsaloum M, Higerd GP, Effraim PR, Waxman SG. Status of peripheral sodium channel blockers for non-addictive pain treatment. Nat Rev Neurol. 2020;16(12):689-705. https://doi.org/10.1038/s41582-020-00415-2
Li Y, North RY, Rhines LD, et al. DRG voltage-gated sodium channel 1.7 is upregulated in paclitaxel-induced neuropathy in rats and in humans with neuropathic pain. J Neurosci. 2018;38(5):1124–1136. https://doi.org/10.1523/JNEUROSCI.0899-17.2017
Wang GJ, Zhang X, Huang L-D, **ao Y. Involvement of the sodium channel Nav1.7 in paclitaxel-induced peripheral neuropathy through ERK1/2 signaling in rats. Curr Neurovasc Res. 2020;17(3):267–274. https://doi.org/10.2174/1567202617666200514113441
Urru M, Muzzi M, Coppi E, et al. Dexpramipexole blocks Nav1.8 sodium channels and provides analgesia in multiple nociceptive and neuropathic pain models. Pain. 2020;161(4):831–841. https://doi.org/10.1097/j.pain.0000000000001774
Goto Y, Hosomi K, Shimokawa T, et al. Pilot study of repetitive transcranial magnetic stimulation in patients with chemotherapy-induced peripheral neuropathy. J Clin Neurosci. 2020;73:101-107. https://doi.org/10.1016/j.jocn.2020.01.020
Kleckner IR, Kamen C, Gewandter JS, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer. 2018;26(4):1019-1028. https://doi.org/10.1007/s00520-017-4013-0
Di Cesare Mannelli L, Pacini A, Micheli L, et al. Astragali radix: could it be an adjuvant for oxaliplatin-induced neuropathy? Sci Rep. 2017;7(1):42021. https://doi.org/10.1038/srep42021
Lucarini E, Micheli L, Trallori E, et al. Effect of glucoraphanin and sulforaphane against chemotherapy-induced neuropathic pain: Kv7 potassium channels modulation by H2 S release in vivo. Phytother Res. 2018;32(11):2226-2234. https://doi.org/10.1002/ptr.6159
Lee D, Kanzawa-Lee G, Knoerl R, Wyatt G, Smith EML. Characterization of internal validity threats to phase III clinical trials for chemotherapy-induced peripheral neuropathy management: a systematic review. Asia Pac J Oncol Nurs. 2019;6(4):318-332. https://doi.org/10.4103/apjon.apjon_14_19
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bae, E.H., Greenwald, M.K. & Schwartz, A.G. Chemotherapy-Induced Peripheral Neuropathy: Mechanisms and Therapeutic Avenues. Neurotherapeutics 18, 2384–2396 (2021). https://doi.org/10.1007/s13311-021-01142-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-021-01142-2