Abstract
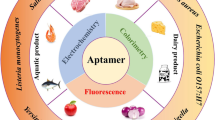
Biosensors are analytical devices for detecting a wide range of targets, including cells, proteins, DNA, enzymes, and chemical and biological compounds. They mostly rely on using bioprobes with a high binding affinity to the target for specific detection. However, low specificity and effectiveness of the conventional biosensors has led to the search for novel materials, that can specifically detect biomolecules. Aptamers are a group of single-stranded DNA or RNA oligonucleotides, that can bind to their targets with high specificity and serve as effective bioprobes for develo** aptamer-based biosensors. Aptamers have a shorter production time, high stability, compared to traditional bioprobes, and possess ability to develop them for specific target molecules for tailored applications. Thus, various aptasensing approaches, including electrochemical, optical, surface plasmon resonance and chip-dependent approaches, have been investigated in recent times for various biological targets, including foodborne pathogens. Hence, this article is an overview of various conventional foodborne pathogen detection methods, their limitations and the ability of aptamer-based biosensors to overcome those limitations and replace them. In addition, the current status and advances in aptamer-based biosensors for the detection of foodborne pathogens to ensure food safety were also discussed.
Similar content being viewed by others
References
Abdul-Mutalib NA, Syafinaz AN, Sakai K, Shirai Y (2015) An overview of foodborne illness and food safety in Malaysia. Int Food Res J 22:896
Adley CC, Ryan MP (2016) The nature and extent of foodborne disease. In: Antimicrobial food packaging, pp. 1–10. Academic Press
Alsager OA, Alotaibi KM, Alswieleh AM, Alyamani BJ (2018) Colorimetric aptasensor of vitamin D3: a novel approach to eliminate residual adhesion between aptamers and gold nanoparticles. Sci Rep 8:12947
Anany H et al (2018) Print to detect: a rapid and ultrasensitive phage-based dipstick assay for foodborne pathogens. Anal Bioanal Chem 410:1217–1230
Arghya S, Suradip D, Pragya S, Utpal B (2012) Aptasensors in health, environment and food safety monitoring. Open J Appl Biosensor. https://doi.org/10.4236/ojab.2012.12002
Aschfalk A, Müller W (2002) Clostridium perfringens toxin types from wild-caught Atlantic cod (Gadus morhua L.), determined by PCR and ELISA. Can J Microbiol 48:365–368
Aslan Y, Atabay M, Chowdhury HK, Göktürk I, Saylan Y, Inci F (2023) Aptamer-based point-of-care devices: emerging technologies and integration of computational methods. Biosensors 13:569
Astill J, Dara RA, Campbell M, Farber JM, Fraser EDG, Sharif S, Yada RY (2019) Transparency in food supply chains: a review of enabling technology solutions. Trends Food Sci Technol 91:240–247
Bacon RT, Sofos JN (2003) Characteristics of biological hazards in foods. Food Safety Handb 10:157–195
Barbau-Piednoir E, Mahillon J, Pillyser J, Coucke W, Roosens NH, Botteldoorn N (2014) Evaluation of viability-qPCR detection system on viable and dead Salmonella serovar Enteritidis. J Microbiol Methods 103:131–137
Berhanu G, Dula TI (2020) Types, importance and limitations of DNA microarray. Glob J Biotechnol Biochem 15:25–31
Bharadwaj S, Dwivedi VD, Kirtipal N (2019) Application of whole genome sequencing (WGS) approach against identification of foodborne bacteria. In: Microbial genomics in sustainable agroecosystems. Springer, pp 131–148
Bhunia AK (2018) Introduction to foodborne pathogens. In: Foodborne microbial pathogens. Springer, pp 1–23
Billington C, Kingsbury JM, Rivas L (2022) Metagenomics approaches for improving food safety: a review. J Food Prot 85:448–464
Bintsis T (2017) Foodborne pathogens. AIMS Microbiol 3:529
Bintsis T (2018) Lactic acid bacteria as starter cultures: an update in their metabolism and genetics. AIMS Microbiol 4:665
Boukharouba A, González A, García-Ferrús M, Ferrús MA, Botella S (2022) Simultaneous detection of four main foodborne pathogens in ready-to-eat food by using a simple and rapid multiplex PCR (mPCR) assay. Int J Environ Res Public Health 19:1031
Buchanan RL, Gorris LGM, Hayman MM, Jackson TC, Whiting RC (2017) A review of Listeria monocytogenes: an update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 75:1–13
Byrne B, Stack E, Gilmartin N, O’Kennedy R (2009) Antibody-based sensors: principles, problems and potential for detection of pathogens and associated toxins. Sensors 9:4407–4445
Cai R, Zhang Z, Chen H, Tian Y, Zhou N (2021) A versatile signal-on electrochemical biosensor for Staphylococcus aureus based on triple-helix molecular switch. Sens Actuators B Chem 326:128842
Cajka T, Vaclavikova M, Dzuman Z, Vaclavik L, Ovesna J, Hajslova J (2014) Rapid LC-MS-based metabolomics method to study the Fusarium infection of barley. J Sep Sci 37:912–919
Cao X et al (2009) Combining use of a panel of ssDNA aptamers in the detection of Staphylococcus aureus. Nucleic Acids Res 37:4621–4628
Celik Uzuner S (2020) Mitochondrial DNA methylation misleads global DNA methylation detected by antibody-based methods. Anal Biochem 601:113789. https://doi.org/10.1016/j.ab.2020.113789
Chao G, Zhou X, Jiao X, Qian X, Xu L (2007) Prevalence and antimicrobial resistance of foodborne pathogens isolated from food products in China. Foodborne Pathog Dis 4:277–284
Chen Y, Wang Z, Shi Q, Huang S, Yu T, Zhang L, Yang H (2021) Multiplex PCR method for simultaneous detection of five pathogenic bacteria closely related to foodborne diseases. 3 Biotech 11:1–8
Chen W, Lai Q, Zhang Y, Liu Z (2022) Recent advances in aptasensors for rapid and sensitive detection of Staphylococcus Aureus. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2022.889431
Cifuentes A (2009) Food analysis and foodomics. J Chromatogr A 1216(43):7109. https://doi.org/10.1016/j.chroma.2009.09.018
Cooper MA (2003) Label-free screening of bio-molecular interactions. Anal Bioanal Chem 377:834–842
Coral-Almeida M, Gabriël S, Abatih EN, Praet N, Benitez W, Dorny P (2015) Taenia solium human cysticercosis: a systematic review of sero-epidemiological data from endemic zones around the world. PLoS Negl Trop Dis 9:e0003919
Costantini F et al (2019) Fluorescent label-free aptasensor integrated in a lab-on-chip system for the detection of ochratoxin a in beer and wheat. ACS Appl Bio Mater 2:5880–5887. https://doi.org/10.1021/acsabm.9b00831
Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB (2013) Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 26:822–880
de Blackburn CW, McClure PJ (2009) Pathogenic Bacillus species. In: Foodborne pathogens. Elsevier, pp 844–888
Desta B (2020) Estimating burden of foodborne diseases where public health impact is higher and data scarcer: a study in four African countries. Eur J Public Health 30:ckaa165-950
Divya DDS, Kumari R, Mahapatra S, Kumar R, Chandra P (2022) Ultrasensitive aptasensors for the detection of viruses based on opto-electrochemical readout systems. Biosensors 12:81
Dkhar DS, Kumari R, Malode SJ, Shetti NP, Chandra P (2022) Integrated lab-on-a-chip devices: fabrication methodologies, transduction system for sensing purposes. J Pharm Biomed Anal 223:115120
Doyle MP, Diez-Gonzalez F, Hill C (2020) Food microbiology: fundamentals and frontiers. John Wiley & Sons, Hoboken
Du X-j, Zhou T-j, Li P, Wang S (2017) A rapid Salmonella detection method involving thermophilic helicase-dependent amplification and a lateral flow assay. Mol Cell Probes 34:37–44
Duan YF, Ning Y, Song Y, Deng L (2014) Fluorescent aptasensor for the determination of Salmonella typhimurium based on a graphene oxide platform. Microchimica Acta 181:647–653
Duan N, Wu S, Dai S, Miao T, Chen J, Wang Z (2015) Simultaneous detection of pathogenic bacteria using an aptamer based biosensor and dual fluorescence resonance energy transfer from quantum dots to carbon nanoparticles. Microchim Acta 182:917–923
Duan N, Chang B, Zhang H, Wang Z, Wu S (2016a) Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int J Food Microbiol 218:38–43
Duan Y, Gao Z, Wang L, Wang H, Zhang H, Li H (2016b) Selection and identification of chloramphenicol-specific DNA aptamers by Mag-SELEX. Appl Biochem Biotechnol 180:1644–1656
Dursun AD, Borsa BA, Bayramoglu G, Arica MY, Ozalp VC (2022) Surface plasmon resonance aptasensor for Brucella detection in milk. Talanta 239:123074. https://doi.org/10.1016/j.talanta.2021.123074
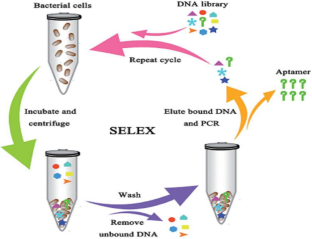
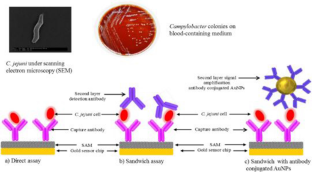
Dwivedi HP, Smiley RD, Jaykus L-A (2010) Selection and characterization of DNA aptamers with binding selectivity to Campylobacter jejuni using whole-cell SELEX. Appl Microbiol Biotechnol 87:2323–2334
E Wang R, Zhang Y, Cai J, Cai W, Gao T (2011) Aptamer-based fluorescent biosensors. Curr Med Chem 18:4175–4184
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Engvall EO, Perlmann P (1972) Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol 109(1):129–135
Fang Z, Wu W, Lu X, Zeng L (2014) Lateral flow biosensor for DNA extraction-free detection of salmonella based on aptamer mediated strand displacement amplification. Biosens Bioelectron 56:192–197
Farka Z, Jurik T, Kovář D, Trnkova L, Skládal P (2017) Nanoparticle-based immunochemical biosensors and assays: recent advances and challenges. Chem Rev 117:9973–10042
Ferone M, Gowen A, Fanning S, Scannell AGM (2020) Microbial detection and identification methods: bench top assays to omics approaches. Compr Rev Food Sci Food Saf 19:3106–3129
Flynn K et al (2019) An introduction to current food safety needs. Trends Food Sci Technol 84:1–3
Foddai ACG, Grant IR (2020) Methods for detection of viable foodborne pathogens: current state-of-art and future prospects. Appl Microbiol Biotechnol 104:4281–4288
Fujimoto Y, Nakamura Y, Ohuchi S (2012) HEXIM1-binding elements on mRNAs identified through transcriptomic SELEX and computational screening. Biochimie 94:1900–1909
Gandhi M, Chikindas ML (2007) Listeria: a foodborne pathogen that knows how to survive. Int J Food Microbiol 113:1–15
Gao X, Jiang T, Qin W (2022) Potentiometric aptasensing of Escherichia coli based on electrogenerated chemiluminescence as a highly sensitive readout. Biosens Bioelectron 200:113923. https://doi.org/10.1016/j.bios.2021.113923
García A, Fox JG, Besser TE (2010) Zoonotic enterohemorrhagic Escherichia coli: a One Health perspective. ILAR J 51:221–232
Gaudreault J, Forest-Nault C, De Crescenzo G, Durocher Y, Henry O (2021) On the use of surface plasmon resonance-based biosensors for advanced bioprocess monitoring. Processes 9:1996
Geng T, Bhunia AK (2006) Optical biosensors in foodborne pathogen detection. In: Smart biosensor technology. CRC Press, pp 527–542
Glynn B, Lahiff S, Wernecke M, Barry T, Smith TJ, Maher M (2006) Current and emerging molecular diagnostic technologies applicable to bacterial food safety. Int J Dairy Technol 59:126–139
Gómez-Govea M, Solís-Soto L, Heredia N, García S, Moreno G, Tovar O, Isunza G (2012) Analysis of microbial contamination levels of fruits and vegetables at retail in Monterrey, Mexico. J Food Agric Environ 10:152–156
Gould LH, Walsh KA, Vieira AR, Herman K, Williams IT, Hall AJ, Cole D (2013) Surveillance for foodborne disease outbreaks—United States, 1998–2008. Morb Mortal Wkly Rep Recomm Rep 62:1–34
Gourama H (2020) Foodborne pathogens. In: Food safety engineering. Springer, pp 25–49
Grace D (2015) Food safety in low and middle income countries. Int J Environ Res Public Health 12:10490–10507
Gribanyov D, Zhdanov G, Olenin A, Lisichkin G, Gambaryan A, Kukushkin V, Zavyalova E (2021) SERS-based colloidal aptasensors for quantitative determination of influenza virus. Int J Mol Sci 22:1842
Grumezescu AM, Holban AM (2018) Food control and biosecurity, vol 16. Academic Press, Cambridge
Hamula CLA, Guthrie JW, Zhang H, Li X-F, Le XC (2006) Selection and analytical applications of aptamers. TrAC Trends Anal Chem 25:681–691
Hamula CLA, Zhang H, Guan LL, Li X-F, Le XC (2008) Selection of aptamers against live bacterial cells. Anal Chem 80:7812–7819
Han D et al (2019) An enzyme-free electrochemiluminesce aptasensor for the rapid detection of Staphylococcus aureus by the quenching effect of MoS2-PtNPs-vancomycin to S2O82−/O2 system. Sens Actuators B Chem 288:586–593
Hayat A, Marty JL (2014) Aptamer based electrochemical sensors for emerging environmental pollutants. Front Chem 2:41
Heredia N, García S (2018) Animals as sources of food-borne pathogens: a review. Anim Nutr 4:250–255
Holban AM, Grumezescu AM (2018) Preface for volume 16: food control and biosecurity. In: Food control and biosecurity. Elsevier, pp. xxiii–xxvi
Holman DB et al (2019) Chlortetracycline enhances tonsil colonization and fecal shedding of multidrug-resistant Salmonella enterica serovar Typhimurium DT104 without major alterations to the porcine tonsillar and intestinal microbiota. Appl Environ Microbiol 85:e02354-e12318
Holmqvist M, Stensjö K, Oliveira P, Lindberg P, Lindblad P (2009) Characterization of the hupSL promoter activity in Nostoc punctiformeATCC 29133. BMC Microbiol 9:1–12
Hong S-L, **ang M-Q, Tang M, Pang D-W, Zhang Z-L (2019) Ebola virus aptamers: from highly efficient selection to application on magnetism-controlled chips. Anal Chem 91:3367–3373
Huang X et al (2020) AIEgens: An emerging fluorescent sensing tool to aid food safety and quality control. Compr Rev Food Sci Food Saf 19:2297–2329
Huang Z, Yu X, Yang Q, Zhao Y, Wu W (2021) Aptasensors for Staphylococcus aureus risk assessment in food. Front Microbiol 12:714265
Hünniger T, Wessels H, Fischer C, Paschke-Kratzin A, Fischer M (2014) Just in time-selection: a rapid semiautomated SELEX of DNA aptamers using magnetic separation and BEAMing. Anal Chem 86:10940–10947
Hurley D et al (2019) Whole-genome sequencing-based characterization of 100 Listeria monocytogenes isolates collected from food processing environments over a four-year period. Msphere 4:e00252-e1219
Hussain MA, Dawson CO (2013) Economic impact of food safety outbreaks on food businesses. Foods 2:585–589
Jagadeesan B et al (2019) The use of next generation sequencing for improving food safety: translation into practice. Food Microbiol 79:96–115
Jaisankar A, Krishnan S, Rangasamy L (2022) Recent developments of aptamer-based lateral flow assays for point-of-care (POC) diagnostics. Anal Biochem 655:114874
Jaklevic MC (2020) FDA expands food safety collaboration with Mexico. JAMA 324:1934–1934
Jauset-Rubio M, El-Shahawi MS, Bashammakh AS, Alyoubi AO, Ciara KO (2017) Advances in aptamers-based lateral flow assays. TrAC Trends Anal Chem 97:385–398
Jemmi T, Stephan R (2006) Listeria monocytogenes: food-borne pathogen and hygiene indicator. Rev Sci Tech 25:571–580
Jia F, Duan N, Wu S, Dai R, Wang Z, Li X (2016) Impedimetric Salmonella aptasensor using a glassy carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim Acta 183:337–344
Khang J, Kim D, Chung KW, Lee JH (2016) Chemiluminescent aptasensor capable of rapidly quantifying Escherichia coli O157: H7. Talanta 147:177–183
Khateb H, Klös G, Meyer RL, Sutherland DS (2020) Development of a label-free LSPR-apta sensor for Staphylococcus aureus detection. ACS Appl Bio Mater 3:3066–3077
Kim JS et al (2007) A novel multiplex PCR assay for rapid and simultaneous detection of five pathogenic bacteria: Escherichia coli O157: H7, Salmonella, Staphylococcus aureus, Listeria monocytogenes, and Vibrio parahaemolyticus. J Food Prot 70:1656–1662
Kolm C et al (2020) DNA aptamers against bacterial cells can be efficiently selected by a SELEX process using state-of-the art qPCR and ultra-deep sequencing. Sci Rep 10:1–16
Kumar BK, Raghunath P, Devegowda D, Deekshit VK, Venugopal MN, Karunasagar I, Karunasagar I (2011) Development of monoclonal antibody based sandwich ELISA for the rapid detection of pathogenic Vibrio parahaemolyticus in seafood. Int J Food Microbiol 145:244–249
Kwol VS, Avci T, Eluwole KK, Dalhatu A (2020) Food safety knowledge and hygienic-sanitary control: a needed company for public well-being. J Public Aff 20:e2067
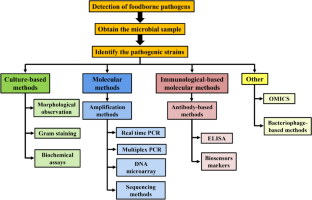
Law JW-F, Ab Mutalib N-S, Chan K-G, Lee L-H (2015) Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol 5:770
Lee H, Yoon Y (2021) Etiological agents implicated in foodborne illness world wide. Food Sci Anim Resour 41:1
Lerdsri J, Chananchana W, Upan J, Sridara T, Jakmunee J (2020) Label-free colorimetric aptasensor for rapid detection of aflatoxin B1 by utilizing cationic perylene probe and localized surface plasmon resonance of gold nanoparticles. Sens Actuators B Chem 320:128356
Liu R et al (2021) Development of a fluorescence aptasensor for rapid and sensitive detection of Listeria monocytogenes in food. Food Control 122:107808. https://doi.org/10.1016/j.foodcont.2020.107808
Liu M et al (2022) Liquid crystal-based optical aptasensor for the sensitive and selective detection of Gram-negative bacteria. Sci China Chem 65:2023–2030. https://doi.org/10.1007/s11426-022-1336-x
Liu J, **e G, Lv S, **ong Q, Xu H (2023) Recent applications of rolling circle amplification in biosensors and DNA nanotechnology. TrAC Trends Anal Chem 160:116953
Long W, Patra I, Rahi Alhachami F, Akhrarovich Sherbekov U, Majdi A, Abed SA (2022) aptamer based nanoprobes for detection of foodborne virus in food and environment samples: recent progress and challenges. Crit Rev Anal Chem. https://doi.org/10.1080/10408347.2022.2114785
Lorenz C, Von Pelchrzim F, Schroeder R (2006) Genomic systematic evolution of ligands by exponential enrichment (Genomic SELEX) for the identification of protein-binding RNAs independent of their expression levels. Nat Protoc 1:2204–2212
Lorenz C et al (2010) Genomic SELEX for Hfq-binding RNAs identifies genomic aptamers predominantly in antisense transcripts. Nucleic Acids Res 38:3794–3808
Lund BM, O’Brien SJ (2011) The occurrence and prevention of foodborne disease in vulnerable people. Foodborne Pathog Dis 8:961–973
Ma Y, Li X, Li W, Liu Z (2018) Glycan-imprinted magnetic nanoparticle-based SELEX for efficient screening of glycoprotein-binding aptamers. ACS Appl Mater Interfaces 10:40918–40926
Mahmoud B (2019) The most common food safety incidents related to develo** countries. Food Safety Magazine 11(1–4). https://www.foodsafetymagazine.com/enewsletter/the-most-common-food-safetyincidents-related-to-develo**-countries/
Majdinasab M, Hayat A, Marty JL (2018) Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trends Anal Chem 107:60–77
Mandal PK, Biswas AK, Choi K, Pal UK (2011) Methods for rapid detection of foodborne pathogens: an overview. Am J Food Technol 6:87–102
Masdor NA, Altintas Z, Tothill IE (2017) Surface plasmon resonance immunosensor for the detection of Campylobacter jejuni. Chemosensors 5:16
McMeekin TA (2003) Detecting pathogens in food. Elsevier, Amsterdam
Meyer C, Fredriksson-Ahomaa M, Sperner B, Märtlbauer E (2011) Detection of Listeria monocytogenes in pork and beef using the VIDAS® LMO2 automated enzyme linked immunoassay method. Meat Sci 88:594–596
Miller S, Chiu C (2022) The role of metagenomics and next-generation sequencing in infectious disease diagnosis. Clin Chem 68:115–124
Mishra RK, Hayat A, Catanante G, Ocaña C, Marty J-L (2015) A label free aptasensor for Ochratoxin A detection in cocoa beans: an application to chocolate industries. Anal Chim Acta 889:106–112
Mulugeta K (2010) Food safety and foodborne disease in 21st century homes. Canada J Infect Dis 14(5):277–280
Nassarawa SS, Luo Z, Lu Y (2022) Conventional and emerging techniques for detection of foodborne pathogens in horticulture crops: a leap to food safety. Food Bioprocess Technol 15:1248–1267
Nehra M, Kumar V, Kumar R, Dilbaghi N, Kumar S (2022) Current scenario of pathogen detection techniques in agro-food sector. Biosensors 12:489
Nguyen TT-Q, Gu MB (2023) An ultrasensitive electrochemical aptasensor using Tyramide-assisted enzyme multiplication for the detection of Staphylococcus aureus. Biosens Bioelectron 228:115199. https://doi.org/10.1016/j.bios.2023.115199
Nguyen HH, Park J, Kang S, Kim M (2015) Surface plasmon resonance: a versatile technique for biosensor applications. Sensors 15:10481–10510
Nikoleli G-P, Siontorou CG, Nikolelis DP, Bratakou S, Karapetis S, Tzamtzis N (2018) Biosensors based on microfluidic devices lab-on-a-chip and microfluidic technology. Nanotechnol Biosens. https://doi.org/10.1016/B978-0-12-813855-7.00013-1
Oliver JD (2010) Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev 34:415–425
Pandhi S, Kumar A, Mishra S (2023) Foodborne diseases: causative agents and related microorganisms. In: Global food safety. Apple Academic Press, pp 39–55
Park L, Kim J, Lee JH (2013) Role of background observed in aptasensor with chemiluminescence detection. Talanta 116:736–742
Pestourie C et al (2006) Comparison of different strategies to select aptamers against a transmembrane protein target. Oligonucleotides 16:323–335
Pires SM et al (2021) Burden of foodborne diseases: think global, act local. Curr Opin Food Sci 39:152–159
Postollec F, Falentin H, Pavan S, Combrisson J, Sohier D (2011) Recent advances in quantitative PCR (qPCR) applications in food microbiology. Food Microbiol 28:848–861
Priyanka B, Patil RK, Dwarakanath S (2016) A review on detection methods used for foodborne pathogens. Indian J Med Res 144:327
Radhakrishnan S, Mathew M, Rout CS (2022) Microfluidic sensors based on two-dimensional materials for chemical and biological assessments. Mater Adv 3:1874–1904
Radhika M, Saugata M, Murali HS, Batra HV (2014) A novel multiplex PCR for the simultaneous detection of Salmonella enterica and Shigella species. Braz J Microbiol 45:667–676
Radi A-E (2011) Electrochemical aptamer-based biosensors: recent advances and perspectives. Int J Electrochem 2011:863196. https://doi.org/10.4061/2011/863196
Radi A-E, Abd-Ellatief MR (2021) Electrochemical aptasensors: current status and future perspectives. Diagnostics 11:104
Richter Ł, Janczuk-Richter M, Niedziółka-Jönsson J, Paczesny J, Hołyst R (2018) Recent advances in bacteriophage-based methods for bacteria detection. Drug Discov Today 23:448–455
Ronholm J (2018) Game changer-Next generation sequencing and its impact on food microbiology, vol 9. Frontiers Media, Lausanne
Rubab M, Shahbaz HM, Olaimat AN, Oh D-H (2018) Biosensors for rapid and sensitive detection of Staphylococcus aureus in food. Biosens Bioelectron 105:49–57
Rudi K, Moen B, Drømtorp SM, Holck AL (2005) Use of ethidium monoazide and PCR in combination for quantification of viable and dead cells in complex samples. Appl Environ Microbiol 71:1018–1024
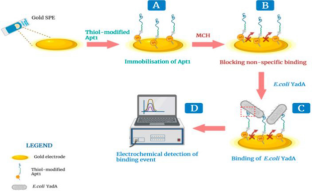
Sande MG et al (2022) Electrochemical aptasensor for the detection of the key virulence factor YadA of Yersinia enterocolitica. Biosensors (basel). https://doi.org/10.3390/bios12080614
Santiago-Felipe S, Tortajada-Genaro LA, Puchades R, Maquieira A (2014) Recombinase polymerase and enzyme-linked immunosorbent assay as a DNA amplification-detection strategy for food analysis. Anal Chim Acta 811:81–87
Saravanan A, Kumar PS, Hemavathy RV, Jeevanantham S, Kamalesh R, Sneha S, Yaashikaa PR (2021) Methods of detection of food-borne pathogens: a review. Environ Chem Lett 19:189–207
Sarojnalini C, Hei A (2019) Fish as an important functional food for quality life u: functional foods. Lagouri, V, Ured, pp. 77–97
Schatz GC, Young MA, Duyne RPV (2006) Electromagnetic mechanism of SERS. In: Surface-enhanced Raman scattering. Springer, pp 19–45
Schmelcher M, Loessner MJ (2014) Application of bacteriophages for detection of foodborne pathogens. Bacteriophage 4:e28137
Sergueev KV, He Y, Borschel RH, Nikolich MP, Filippov AA (2010) Rapid and sensitive detection of Yersinia pestis using amplification of plague diagnostic bacteriophages monitored by real-time PCR. PLoS ONE 5:e11337
Shamah SM, Healy JM, Cload ST (2008) Complex target SELEX. Acc Chem Res 41:130–138
Sheng L, Wang L (2021) The microbial safety of fish and fish products: recent advances in understanding its significance, contamination sources, and control strategies. Compr Rev Food Sci Food Saf 20:738–786
Shrivastava S, Lee W-I, Lee N-E (2018) Culture-free, highly sensitive, quantitative detection of bacteria from minimally processed samples using fluorescence imaging by smartphone. Biosens Bioelectron 109:90–97
Smolsky J, Kaur S, Hayashi C, Batra SK, Krasnoslobodtsev AV (2017) Surface-enhanced Raman scattering-based immunoassay technologies for detection of disease biomarkers. Biosensors 7:7
Song Z, Zhao Z, Ren W, He B (2023) Aptamer-based colorimetric and lateral flow chromatographic strip detection of Aflatoxin B 1 in corn samples. https://doi.org/10.21203/rs.3.rs-2667935/v1
Sorrenti V, Di Giacomo C, Acquaviva R, Barbagallo I, Bognanno M, Galvano F (2013) Toxicity of ochratoxin A and its modulation by antioxidants: a review. Toxins 5:1742–1766
Sousa CPd (2008) The impact of food manufacturing practices on food borne diseases. Braz Arch Biol Technol 51:615–623
Stein RA, Chirilã M (2017) Routes of transmission in the food chain. In: Foodborne diseases. Elsevier, pp 65–103
Suliman Maashi M (2023) CRISPR/Cas-based aptasensor as an innovative sensing approaches for food safety analysis: recent progresses and new horizons. Crit Rev Anal Chem. https://doi.org/10.1080/10408347.2023.2188955
Tao J et al (2020) A multiplex PCR assay with a common primer for the detection of eleven foodborne pathogens. J Food Sci 85:744–754
Tao X, Liao Z, Zhang Y, Fu F, Hao M, Song Y, Song E (2021) Aptamer-quantum dots and teicoplanin-gold nanoparticles constructed FRET sensor for sensitive detection of Staphylococcus aureus. Chin Chem Lett 32:791–795
Teng J et al (2016) Aptamer-based technologies in foodborne pathogen detection. Front Microbiol 7:1426
Tian C, Zhao L, Qi G, Zhang S (2023) Trace detection of E. coli O157:H7 cells by an Au nanoparticle-based SERS aptasensor. ACS Appl Nano Mater 6:1386–1394. https://doi.org/10.1021/acsanm.2c05031
Touron A, Berthe T, Pawlak B, Petit F (2005) Detection of Salmonella in environmental water and sediment by a nested-multiplex polymerase chain reaction assay. Res Microbiol 156:541–553
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Umesha S, Manukumar HM (2018) Advanced molecular diagnostic techniques for detection of food-borne pathogens: current applications and future challenges. Crit Rev Food Sci Nutr 58:84–104
Velusamy V, Arshak K, Korostynska O, Oliwa K, Adley C (2010) An overview of foodborne pathogen detection: In the perspective of biosensors. Biotechnol Adv 28:232–254
Voelkerding KV, Dames SA, Durtschi JD (2009) Next-generation sequencing: from basic research to diagnostics. Clin Chem 55:641–658
Wang C et al (2003) Single-stranded DNA aptamers that bind differentiated but not parental cells: subtractive systematic evolution of ligands by exponential enrichment. J Biotechnol 102:15–22
Wang W-W, Han X, Chu L-Q (2019) Polyadenine-mediated immobilization of aptamers on gold substrate for direct detection of bacterial pathogens. Anal Sci. https://doi.org/10.2116/analsci.19P110
Wang M, Zhang Y, Tian F, Liu X, Du S, Ren G (2021) Overview of rapid detection methods for Salmonella in foods: progress and challenges. Foods 10:2402
Wen J-D, Gray DM (2004) Selection of genomic sequences that bind tightly to Ff gene 5 protein: primer-free genomic SELEX. Nucleic Acids Res 32:e182–e182
Weng X, Neethirajan S (2016) A microfluidic biosensor using graphene oxide and aptamer-functionalized quantum dots for peanut allergen detection. Biosens Bioelectron 85:649–656
Weng X, Neethirajan S (2017) Aptamer-based fluorometric determination of norovirus using a paper-based microfluidic device. Microchim Acta 184:4545–4552
Weng X, Neethirajan S (2018) Paper-based microfluidic aptasensor for food safety. J Food Saf 38:e12412
World Health O (2015) WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference grou. World Health Organization, Geneva, pp 2007–2015
Wu W, Zhao S, Mao Y, Fang Z, Lu X, Zeng L (2015) A sensitive lateral flow biosensor for Escherichia coli O157: H7 detection based on aptamer mediated strand displacement amplification. Anal Chim Acta 861:62–68
Xu L, Callaway ZT, Wang R, Wang H, Slavik MF, Wang A, Li Y (2015) A fluorescent aptasensor coupled with nanobead-based immunomagnetic separation for simultaneous detection of four foodborne pathogenic bacteria. Trans ASABE 58:891–906
Xu Y, Jiang X, Zhou Y, Ma M, Wang M, Ying B (2021) Systematic evolution of ligands by exponential enrichment technologies and aptamer-based applications: recent progress and challenges in precision medicine of infectious diseases. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2021.704077/full
Yan X-L, Xue X-X, Luo J, Jian Y-T, Tong L, Zheng X-J (2020) Construction of chemiluminescence aptasensor platform using magnetic microsphere for ochratoxin A detection based on G bases derivative reaction and Au NPs catalyzing luminol system. Sens Actuators B Chem 320:128375
Yi J et al (2019) A composite prepared from carboxymethyl chitosan and aptamer-modified gold nanoparticles for the colorimetric determination of Salmonella typhimurium. Microchim Acta 186:1–8
Yu M et al (2017) Dual-recognition förster resonance energy transfer based platform for one-step sensitive detection of pathogenic bacteria using fluorescent vancomycin-gold nanoclusters and aptamer-gold nanoparticles. Anal Chem 89:4085–4090
Yuan R, Cai J, Ma H, Luo Y, Wang L, Su S (2023) Recent progress in electrochemical aptasensors: construction and application. Chemosensors 11:488
Zahra Q, Khan QA, Luo Z (2021) Advances in optical aptasensors for early detection and diagnosis of various cancer types. Front Oncol 11:632165
Zavyalova E et al (2021) SERS-based aptasensor for rapid quantitative detection of SARS-CoV-2. Nanomaterials 11:1394
Zeng D, Chen Z, Jiang Y, Xue F, Li B (2016) Advances and challenges in viability detection of foodborne pathogens. Front Microbiol 7:1833
Zeng L, Wang L, Hu J (2018) Current and emerging technologies for rapid detection of pathogens. Biosens Technol Detect Pathog Prospect Way Anal 73178:6–19
Zhang G, Zhu C, Huang Y, Yan J, Chen A (2018) A lateral flow strip based aptasensor for detection of ochratoxin A in corn samples. Molecules 23:291
Zhang J, Zhang B, Wu Y, Jia S, Fan T, Zhang Z, Zhang C (2010) Fast determination of the tetracyclines in milk samples by the aptamer biosensor. Analyst 135:2706–2710
Zhang H, Ma X, Liu Y, Duan N, Wu S, Wang Z, Xu B (2015) Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens Bioelectron 74:872–877
Zhang Z, Zhou J, Du X (2019) Electrochemical biosensors for detection of foodborne pathogens. Micromachines 10:222
Zhang T, Liu J, Zhang L, Irfan M, Su X (2023) Recent advances in the aptamer-based biosensors for potassium detection. Analyst 48(21):5340–5354. https://doi.org/10.1039/D3AN01053H
Zhao X, Lin C-W, Wang J, Oh DH (2014) Advances in rapid detection methods for foodborne pathogens. J Microbiol Biotechnol 24:297–312
Zhou B et al (2022) Novel species-specific targets for real-time PCR detection of four common pathogenic Staphylococcus spp. Food Control 131:108478
Zhu S, Tang Y, Shi B, Zou W, Wang X, Wang C, Wu Y (2021) Oligonucleotide-mediated the oxidase-mimicking activity of Mn3O4 nanoparticles as a novel colorimetric aptasensor for ultrasensitive and selective detection of Staphylococcus aureus in food. Sens Actuators B Chem 349:130809. https://doi.org/10.1016/j.snb.2021.130809
Acknowledgements
MKD and TABT wish to acknowledge the support of the National Science Foundation Grant (#2130658) for their contribution to this work. All the other authors acknowledge their respective universities and departments for their support during the preparation of this manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
TAB-T, SB, RRK, GO-B, CA—Preparation of initial draft, JJ, DA, MKD—Revision.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bruce-Tagoe, T.A., Bhaskar, S., Kavle, R.R. et al. Advances in aptamer-based biosensors for monitoring foodborne pathogens. J Food Sci Technol 61, 1252–1271 (2024). https://doi.org/10.1007/s13197-023-05889-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05889-8