Abstract
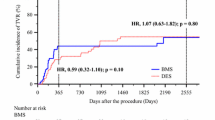
To investigate the clinical outcomes after biodegradable-polymer (BP) and durable-polymer (DP) everolimus-eluting stent (EES) implantation in hemodialysis (HD) patients with coronary artery disease. We enrolled 221 consecutive HD patients successfully treated with EES implantation for coronary lesions. Over the following 2 years, we assessed the incidence of target lesion revascularization (TLR) and major adverse cardiac event (MACE), defined as the composite endpoint of TLR, all-cause mortality, or myocardial infarction. We performed a propensity-score matching analysis and collected follow-up coronary angiography data. There were 91 patients in the BP-EES group and 130 in the DP-EES group. Male sex and diabetes rates were significantly lower in the BP-EES group than in the DP-EES group. A debulking device was less frequently used in the BP-EES group than in the DP-EES group (7.6% vs. 21.5%, p = 0.006). TLR occurred in 38 patients, while stent thrombosis was observed in 3 patients; 19 patients died. TLR and MACE rates at 2 years were comparable between the two groups (19.2% in the BP-EES group vs. 20.4% in the DP-EES group, p = 0.73 and 26.9% vs. 34.2%, p = 0.93, respectively). In the propensity-score-matched cohort, TLR and MACE rates were similar between the two groups (19.2% in the BP-EES group vs. 18.1% in the DP-EES group, p = 0.69, and 26.9% vs. 30.2%, p = 0.66, respectively). Restenosis rates at follow-up angiography were similar between the two groups (p = 0.79). In hemodialysis patients, BP-EES and DP-EES showed similar 2-year clinical outcomes.


Similar content being viewed by others
References
Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO study. Kidney Int. 2004;65:2380–9.
Ting HH, Tahirkheli NK, Berger PB, McCarthy JT, Timimi FK, Mathew V, et al. Evaluation of long-term survival after successful percutaneous coronary intervention among patients with chronic renal failure. Am J Cardiol. 2001;87:630–3.
Yasuda K, Kasuga H, Aoyama T, Takahashi H, Toriyama T, Kawade Y, et al. Comparison of percutaneous coronary intervention with medication in the treatment of coronary artery disease in hemodialysis patients. J Am Soc Nephrol. 2006;17:2322–32.
Nakazawa G, Tanabe K, Aoki J, Yamamoto H, Higashikuni Y, Onuma Y, et al. Impact of renal insufficiency on clinical and angiographic outcomes following percutaneous coronary intervention with sirolimus-eluting stents. Catheter Cardiovasc Interv. 2007;69:808–14.
Aoyama T, Ishii H, Toriyama T, Takahashi H, Kasuga H, Murakami R, et al. Sirolimus-eluting stents vs bare metal stents for coronary intervention in Japanese patients with renal failure on hemodialysis. Circ J. 2008;72:56–60.
Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, Goedhart D, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201–9.
Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362:1663–74.
Sakakibara T, Ishii H, Toriyama T, Aoyama T, Takahashi H, Kamoi D, et al. Sirolimus-eluting stent vs. everolimus-eluting stent for coronary intervention in patients on chronic hemodialysis. Circ J. 2012;76:351–5.
Ikari Y, Kyono H, Isshiki T, Ishizuka S, Nasu K, Sano K, et al. Usefulness of everolimus-eluting coronary stent implantation in patients on maintenance hemodialysis. Am J Cardiol. 2015;116:872–6.
Kereiakes DJ, Meredith IT, Windecker S, Lee Jobe R, Mehta SR, Sarembock IJ, et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II randomized trial. Circ Cardiovasc Interv. 2015;8:e002372.
Kereiakes DJ, Windecker S, Jobe RL, Mehta SR, Sarembock IJ, Feldman RL, et al. Clinical outcomes following implantation of thin-strut, bioabsorbable polymer-coated, everolimus-eluting SYNERGY stents. Circ Cardiovasc Interv. 2019;12:e008152.
Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB 3rd, Loop FD, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on assessment of diagnostic and therapeutic cardiovascular procedures (subcommittee on percutaneous transluminal coronary angioplasty). Circulation. 1988;78:486–502.
Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959–65.
Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–8.
Otsuka M, Shiode N, Masaoka Y, Okimoto T, Tamekiyo H, Kawase T, et al. Comparison of everolimus- and paclitaxel-eluting stents in dialysis patients. Cardiovasc Revasc Med. 2015;16:208–12.
Masakane I, Taniguchi M, Nakai S, Tsuchida K, Wada A, Ogata S, et al. Annual dialysis data report 2016, JSDT renal data registry. Ren Replace Ther. 2018;4:45.
Shreenivas SS, Kereiakes DJ. Evolution of the SYNERGY bioresorbable polymer metallic coronary stent. Future Cardiol. 2018;14:307–17.
Kereiakes DJ. Healing by design: in vivo insights following contemporary coronary stent deployment. Eur Heart J. 2018;39:2457–9.
Sato T, Hatada K, Kishi S, Fuse K, Fujita S, Ikeda Y, et al. Comparison of clinical outcomes of coronary artery stent implantation in patients with end-stage chronic kidney disease including hemodialysis for three everolimus eluting (EES) stent designs: bioresorbable polymer-EES, platinum chromium-EES, and cobalt chrome-EES. J Interv Cardiol. 2018;31:170–6.
Natsuaki M, Kozuma K, Morimoto T, Kadota K, Muramatsu T, Nakagawa Y, et al. Biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2013;62:181–90.
Shiomi H, Kozuma K, Morimoto T, Igarashi K, Kadota K, Tanabe K, et al. Long-term clinical outcomes after everolimus- and sirolimus-eluting coronary stent implantation: final 3-year follow-up of the randomized evaluation of sirolimus-eluting versus everolimus-eluting stent trial. Circ Cardiovasc Interv. 2014;7:343–54.
Fujimoto Y, Kobayashi Y, Kato K, Yamaguchi M. Delamination of novel ultrathin bioabsorbable abluminal polymer of platinum chromium everolimus-eluting stent. Cardiovasc Interv Ther. 2018;33:97–8.
**nouchi H, Kutyna M, Torii S, Cheng Q, Sakamoto A, Guo L, et al. Comparison of acute thrombogenicity and albumin adsorption in three different durable polymer coronary drug-eluting stents. EuroIntervention. 2021;17:248–56.
Sato Y, **nouchi H, Kolodgie FD, Cheng Q, Janifer C, Kutyna M, et al. Acute thrombogenicity of fluoropolymer coated stents versus competitive drug-eluting stents under single antiplatelet therapy. Int J Cardiol. 2021;338:42–9.
Torii S, Cheng Q, Mori H, Lipinski MJ, Acampado E, Perkins LEL, et al. Acute thrombogenicity of fluoropolymer-coated versus biodegradable and polymer-free stents. EuroIntervention. 2019;14:1685–93.
Palmerini T, Barozzi C, Tomasi L, Riva DD, Marengo M, Cicoria G, et al. In vitro thrombogenicity of drug-eluting and bare metal stents. Thromb Res. 2020;185:43–8.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: RI and TS; methodology: SO and TN; formal analysis and investigation: RI and MK; writing—original draft preparation: RI; writing—review and editing: HI; supervision: TM.
Corresponding author
Ethics declarations
Conflict of interest
H.I. received lecture fees from Astellas Pharma Inc. Daiichi-Sankyo Pharma Inc. and MSD K.K. T.M. received lecture fees from Bayel Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Kowa Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Pfizer Japan Inc., Sanofi-aventis K.K., and Takeda Pharmaceutical Co., Ltd. T.M. received unrestricted research grant for Department of Cardiology, Nagoya University Graduate School of Medicine from Astellas Pharma Inc, Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Kowa Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Otsuka Pharma Ltd., Pfizer Japan Inc., Sanofi-aventis K.K., Takeda Pharmaceutical Co., Ltd., Tei** Pharma Ltd.
Ethical approval
This retrospective study was approved by the institutional review board of Nagoya Kyoritsu Hospital and was conducted in accordance with the Helsinki Declaration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ito, R., Ishii, H., Oshima, S. et al. Comparison between biodegradable- and durable-polymer everolimus-eluting stents in hemodialysis patients with coronary artery disease. Cardiovasc Interv and Ther 37, 475–482 (2022). https://doi.org/10.1007/s12928-021-00827-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-021-00827-x