Abstract
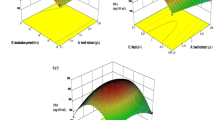
Agricultural, fruit and vegetable wastes were investigated as cost effective source for pectinase production. Orange peel proved as the best inducer of pectinase in a very simple culture medium used. Optimization of various cultural parameters was carried out by statistical method using response surface methodology and central composite design. The basic aim was to find out the best among local agri-wastes for maximum production of pectinase kee** in mind the agro-based economy of the country. Also the aim was to find the best pectinase of alkaline and thermophilic nature having maximum activity in minimum time, to be used in various industrial processes mainly textile industry which is the key industry in a country like Pakistan. The Bacillus licheniformis used in the present study was of special worth as it gave maximum pectinase activity of 219 U/ml in submerged fermentation, in a very simple medium with very few components (NaNO3, KH2PO4, KCl, MgSO4, Tryptone and Orange peel) under optimum conditions of pH 9.5, when incubated at 37 °C for 120 h in an alkaline culture medium of pH 9.5 supplementing with 0.3 % inoculum, 2.5 % orange peel and 0.5 % tryptone. The enzyme exhibited thermophilic nature by showing maximum activity when incubated with substrate (0.5 % citrus pectin solution pH 8.0) at 70 °C for 10 min. The study proved that orange peel has nutrients that enabled the microorganism understudy to produce high quantity (219 U/ml) of pectinase in very simple medium and can be better option for pectinase production on large scale for commercial use in industries.













Similar content being viewed by others
References
Jayani, R.S., Saxena, S., Gupta, R.: Microbial pectinolytic enzymes: a review. Process Biochem. 9, 2931–2944 (2005)
Sarrouh, B., Santos, T.M., Miyoshi, A., Dias, R., Azevedo, V.: Up-to-date insight on industrial enzymes applications and global market. J. Bioprocess. Biotech. 4, 1–10 (2012)
Dewan, S.S.: Global Markets for Enzymes in Industrial Applications, Report Overview BIO030G (2014)
Ghani, M., Ansari, A., Aman, A., Zohra, R.R., Siddiqui, N.N., Qader, S.A.U.: Isolation and characterization of different strains of Bacillus licheniformis for the production of commercially significant enzymes. Pak. J. Pharm. Sci. 26, 691–697 (2013)
Qureshi, A.S., Bhutto, M.A., Chisti, Y., Khushk, I., Dahot, M.U., Bano, S.: Production of pectinase by Bacillus subtilis EFRL 01 in a date syrup medium. Afr. J. Biotechnol. 11, 12563–12570 (2012)
Ajit, K., Rita, S.: Production of alkaline pectinase by bacteria (Cocci sps.) isolated from decomposing fruit materials. J. Phytol. 4, 01–05 (2012)
Martos, M.A., Zubreski, E.R., Garro, O.A., Hours, R.A.: Production of Pectinolytic enzymes by the yeast Wickerhanomyces anomalus isolated from citrus fruits peels. Biotechnol. Res. Int. 2013, 1–7 (2013)
Merín, M.G., Mendoza, L.M., Morata de Ambrosini, V.I.: Pectinolytic yeasts from viticultural and enological environments: novel finding of Filobasidium capsuligenum producing pectinases. J. Basic Microbiol. 54, 835–842 (2014)
Göğüş, N., HakgüderTaze, B., DEMİR, H., Tari, C., Ünlütürk, S., Lahore, M.F.: Evaluation of orange peel, an industrial waste, for the production of Aspergillus sojae polygalacturonase considering both morphology and rheology effects. Turk. J. Biol. 38, 537–548 (2014)
Yadav, K.K., Garg, N., Kumar, D., Kumar, S., Singh, A., Mutukumar, M.: Application of response surface methodology for optimization of polygalacturonase production by Aspergillus niger. J. Environ. Boil. 36, 255–259 (2015)
Martos, M.A., Zubreski, E.R., Combina, M., Garro, O.A., Hours, R.A.: Isolation of a yeast strain able to produce a polygalacturonase with maceration activity of cassava roots. Food Sci. Technol. 33, 332–338 (2013)
Mehrnoush, A., Mustafa, S., Yazid, A.M.M.: Characterization of pectinase from mango (Mangifera indica cv. Chokanan) peel. J. Food Agric. Environ. 10, 85–88 (2012)
Ramirez, H.L., Gómez Brizuela, L., Úbeda Iranzo, J., Arevalo-Villena, M., Briones Pérez, A.I.: Pectinase immobilization on a chitosan-coated chitin support. J. Food Process Eng. 39, 97–104 (2015)
Thangaratham, T., Manimegalai, G.: Optimization and production of pectinase using agro waste by solid state and submerged fermentation. Int. J. Curr. Microbiol. App. Sci. 3, 357–365 (2014)
Mojsov, K., Ziberoski, J., Bozinovic, Z., Petreska, M.: A comparison of effects of three commercial pectolytic enzyme preparations in red wine making. Int. J. Pure Appl. Sci. Technol. 1, 127–136 (2010)
Sieiro, C., García-Fraga, B., López-Seijas, J., Silva, A.F. Da., Villa, T.G.: Microbial pectic enzymes in the food and wine industry. In: B. Valdez (ed.) Food Industrial Processes - Methods and Equipment, pp. 201–218 (2012). doi:10.5772/33403
Ajayi, A.A., Osunkoya, F.A., Peter-Albert, C.F., Olasehinde, G.I.: Clarification of apple juice with laboratoryproduced-pectinase obtained from the deteriorationof apple (Malus domestica) fruits by Aspergillus niger. Intl. J. Adv. Biotechnol. Res. 5, 134–140 (2014)
Sharma, H.P., Patel, H., Sharma, S.: Enzymatic extraction and clarification of juice from various fruits—a review. Trends Post Harvest Technol. 2, 01–14 (2014)
Uçan, F., Akyildiz, A., Erdal, A.L.: Effect of different enzymes and concentrations in the production of clarified lemon juice. J. Food Process. 2014, 14 (2014). doi:10.1155/2014/215854
Sharma, D.C., Satyanarayana, T.: Biotechnological potential of agro residues for economical production of thermoalkali-stable pectinase by Bacillus pumilus dcsr1 by solid-state fermentation and its efficacy in the treatment of ramie fibres. Enzyme Res. 2012, 7 (2012). doi:10.1155/2012/281384
Zhang, C., Yao, J., Zhou, C., Mao, L., Zhang, G., Yanhe, M.: The alkaline pectate lyase PEL168 of Bacillus subtilis heterologously expressed in Pichia pastoris is more stable and efficient for Degumming ramie fiber. BMC Biotechnol. 13, 26 (2013)
Tanabe, H., Yoshihara, Y., Tamura, K., Kobayashi, Y., Terashita, T., Sakai, T.: Preteatment of pectic waste water from orange canning process by an alkalophilic Bacillus sp. J. Ferment. Technol. 65, 243–246 (1987)
Murthy, P.S., Naidu, M.M.: Improvement of robusta coffee fermentation with microbial enzymes. Eur. J. Appl. Sci. 3, 130–139 (2011)
Mojtaba, A., Fardin, K.: Optimization of enzymatic extraction of oil from Pistacia Khinjuk seeds by using central composite design. Food Sci. Technol. 1, 37–43 (2013)
Mortabit, D., Mourad Zyani, M., Koraichi, S.I.: Improving olive oil yield from Moroccan Picholine by bacterial enzymes extract. IJISET 1, 717–728 (2014)
Perez, E.E., Fernández, M.B., Nolasco, S.M., Crapiste, G.H.: Effect of pectinase on the oil solvent extraction from different genotypes of sunflower (Helianthus annuus L.). J. Food Eng. 117, 393–398 (2013)
Dzogbefia, V.P., Ofosu, G.A., Oldham, J.H.: Evaluation of locally produced Saccharomyces cerevisiae pectinase enzyme for industrial extraction of starch from cassava in Ghana. Sci. Res. Essays 3, 365–369 (2008)
Wilkins, M.R., Widmer, W.W., Grohmann, K.: Simultaneous Saccharafication and Fermentation of citrus peel waste by Sacchromyces cerevisiea to produce ethanol. Process Biochem. 42, 1614–1619 (2007)
Altaf, N., Khan, A.R.: Growth and development of low seeded Kinnow mandarin fruits in dense plantation. J. Agric. Sci. Technol. 11, 191–198 (2009)
Diaz, A.B., Ory, I.D., Caro, I., Belandino, A.: Production of hydrolytic enzymes from grape pomace and orange peels mixed susbstrate fermentation by Aspergillus awamori. Chem. Eng. Trans. 17, 1143–1148 (2009)
Kumar, P.G., Suneetha, V.: Natural, culinary fruit peels as a potential substrate for pectinolytic enzyme int. J. Drug Dev. Res. 6, 109–118 (2014)
Anvari, M., Khayati, G.: The effect of citrus pulp type on pectinase production in solid-state fermentation: process evaluation and optimization by Taguchi design of experimental (DOE) methodology. J. Biosci. Biotech. 3, 227–233 (2014)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Jagiasi, S.R.: Production of bacterial pectinase from agro-industrial wastes. National conference on biodiversity: status and challenges in conversion. FAVEO (2013)
Mandhania, S., Jain, V., Malhotra, S.P.: Culture optimization for enhanced production of microbial pectin methylesterase under submerged conditions. Asian J. Biotech. 5, 12–22 (2010)
Prakash, S., Karthik, R., M, T.V., Sridhar, B., Bharath, P.G.: Optimization and production of pectinase from Bacillus subtilis (mtcc 441) by using orange peel as a substrate. Int. J. Recent Sci. Res. 5, 1177–1179 (2014)
Motwani, D.R., Meshram, V.G., Jambhulkar, V.S.: Partial purification of pectinase produced by Aspergillus niger grown on wheat bran. IJSER 4, 345–365 (2013)
Patil, R.C., Murugkar, T.P., Shaikh, S.A.: Extraction of pectinase from pectinolytic bacteria isolated from carrot waste. Int. J. Pharma Bio Sci. 3, 261–266 (2012)
Kumari, B.L., Sudhakar, P., Hemamalini, K., Satya sree, N., Vijetha, P.: Studies on pectinase production by Bacillus Subtilis using agro-industrial wastes. RJPBCS 5, 330–339 (2014)
Patil, S.R., Dayanand, A.: Exploration of regional agrowastes for the production of pectinase by Aspergillus niger. Food Technol. Biotechnol. 44, 289–292 (2006)
Oyeleke, S., Oyewole, O., Egwim, E., Dauda, B., Ibeh, E.: Cellulase and pectinase production potentials of Aspergillus Niger isolated from corn cob. Bayero J. Pure Appl. Sci. 5, 78–83 (2012)
Irshad, M., Anwar, Z., Mahmood, Z., Aqil, T., Mehmmod, S., Nawaz, H.: Bio-processing of agro-industrial waste orange peel for induced production of pectinase by Trichoderma viridi; its purification and characterization. Turk. J. Biochem. 39, 9–18 (2014)
Neagu, D.A., Destain, J., Thonart, P., Socaciu, C.: Effects of different carbon sources on pectinase production by Penicillium oxalicum. Bull. UASVM Agric. 69, 327–333 (2012)
Dinarvand, M., Rezaee, M., Masomian, M.: Effect of C/N ratio and media optimization through response surface methodology on simultaneous productions of intra- and extracellular inulinase and invertase from Aspergillus niger ATCC 20611. BioMed Res. Int. 2013(Article ID 508968), 13 (2013)
Embaby, A.M., Masoud, A.A., Marey, H.S., Shaban, N.Z., Ghonaim, T.M.: Raw agro-industrial orange peel waste as a low cost effective inducer for alkaline polygalacturonase production from Bacillus licheniformis SHG10. Springerplus. 3, 327 (2014)
Montgomery, D.C.: Design and Analysis of Experiments: Response Surface Method and Designs. Wiley, New York (2005)
Oehlert, G.W.: Design and Analysis of Experiments: Response Surface Design. Freeman and Company, New York (2000)
Chaturvedi, S., Kohli, K.U., Rajni, S., Khurana, S.M.P.: Statistical optimization of medium composition for Xylanase production by solid state fermentation using Agroresidues. Am. J. Microbiol. Res. 3, 85–92 (2015)
Dave, B.R., Parmar, P., Sudhir, A., Panchal, K., Subramanian, R.B.: Optimization of process parameters for cellulase production by Bacillus licheniformis MTCC 429 using RSM and molecular characterization of cellulase gene. J. Bioprocess. Biotech. 5, 212 (2015). doi:10.4172/2155-9821.1000212
Bhunia, B., Dutta, D., Chaudhuri, S.: Extracellular alkaline protease from Bacillus licheniformis NCIM-2042: improving enzyme activity assay and characterization. Eng. Life Sci. 11, 207–215 (2011)
Sangeetha, R., Geetha, A., Arulpandi, I.: Concomitant production of protease and lipase by Bacillus licheniformis VSG1: production, purification and characterization. Braz. J. Microbiol. 41, 179–185 (2010) ISSN 1517-8382
Kutner, M., Nachtsheim, C., Neter, J., Li, W.: Applied Linear Statistical Models. McGraw-Hill, New York (2005)
Mei, Y., Chena, Y., Zhai, R., Liu, Y.: Cloning, purification and biochemical properties of a thermostable pectinase from Bacillus halodurans M29. J. Mol. Catal. B Enzym. 94, 77–81 (2013)
Rehman, H.U., Qader, S.A.U., Aman, A.: Polygalacturonase: production of pectin depolymerising enzyme from Bacillus licheniformis KIBGE IB-21. Carbohydr. Polym. 90, 387–391 (2012)
Rahman, R.N.Z.A., Lee, P.G., Basri, M., Salleh, A.B.: Phisical factors affecting the production of organic solvent- tolarent protease by Pseudomonas aeruginosa strain K. Bioresour. Technol. 96, 429–436 (2005)
Pranaw, K., Singh, S., Dutta, D., Chaudhuri, S., Ganguly, S., Nain, L.: Statistical optimization of media components for production of fibrinolytic alkaline metalloproteases from Xenorhabdus indica KB-3. Biotechnol. Res. Int. 2014, 11 (2014). doi:10.1155/2014/293434
Bezerra, M.A., Santelli, R.E., Oliveira, E.P., Villar, L.S., Escaleira, L.A.: Response surface methodology (RSM) as a tool for optimization in analytical chemistry. A Rev. Talanta 76, 965–977 (2008)
Acknowledgments
I am highly thankful to Dr. Romana Tabassam, Deputy Chief Scientist in Industrial Biotechnology Division NIBGE, Faisalabad. The entire study was carried out in her supervision. Also many thanks to Higher Education Commission, Pakistan for grant of fellowship for smooth conductance of study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bibi, N., Ali, S. & Tabassum, R. Statistical Optimization of Pectinase Biosynthesis from Orange Peel by Bacillus licheniformis Using Submerged Fermentation. Waste Biomass Valor 7, 467–481 (2016). https://doi.org/10.1007/s12649-015-9470-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-015-9470-4