Abstract
We compared the time-course of satellite cell (SC) activation between eccentric and concentric contractions in the vastus lateralis (VL) muscle after step exercise. Young adults participated in a 30-min step up/down exercise which mainly involved concentric contractions with the right VL muscle and eccentric contractions with the left VL muscle. The concentric and eccentric contraction phases of the VL muscles were identified by changes in the electromyogram (EMG) and knee joint angle. Biopsy samples were taken from both VL muscles at three time periods: before the exercise and 2 and 5 days after the exercise. We found that the numbers of SCs were significantly increased in the type IIa fibers of the left VL at 2 and 5 days after the exercise. The expression of both hepatocyte growth factor (HGF) and myogenic differentiation 1 (MyoD) mRNA had significantly increased in the left VL at 2 and 5 days after the exercise and in the right VL at 5 days after the exercise. The expression of transient receptor potential canonical (TRPC) 1 mRNA also increased in the left VL at 2 days after exercise. These results indicate that eccentric contraction can effectively activate SC proliferation for up to 5 days after exercise. Similar changes in HGF, MyoD and TRPC1 mRNA expression suggest that HGF/c-Met signal activation through cation influx has a major impact on skeletal muscle SC activation in response to eccentric exercise.
Similar content being viewed by others
Introduction
Dynamic muscle contractions can be divided into two types: concentric contractions involving the shortening of muscle fibers and eccentric contractions involving the lengthening of muscle fibers. Eccentric contractions have been shown to possess several physiological properties that distinguish them from concentric contractions. For example, eccentric contraction training results in greater gains in strength and muscle hypertrophy than concentric contraction training at the same metabolic intensity [1–4]. Furthermore, the possibility that eccentric contractions are associated with selective recruitment of fast twitch fibers has been demonstrated [5–7].
Muscle repair and regeneration following injury is dependent on the activation of muscle satellite cells (SCs) which are then released, proliferate and terminally differentiate. SCs are muscle-specific stem cells located under the basal lamina of muscle fibers [8]. In the uninjured adult muscle, SCs remain in a quiescent state for most of the time. However, in response to injury and hypertrophic signals, the cells re-enter the cell cycle, during which they proliferate and differentiate to provide additional myonuclei to existing fibers [9, 10]. The paired box transcription factor 7 (Pax7) is expressed in both quiescent and activated SCs and directs the induction of target genes, such as myogenic differentiation 1 (MyoD), that regulate entry of SCs into the myogenic program [11, 12]. The proliferative phase of the myogenic program is associated with the coexpression of MyoD [13, 14]. The subsequent downregulation of MyoD is associated with the induction of differentiation, while the upregulation of myogenin is known to direct terminal differentiation.
It has been suggested that there is a temporal association between SC regulators, such as insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor (HGF), and the expression of muscle regulatory factors in human tissue after eccentric exercise [15, 16]. In addition, it has been suggested that extracellular Ca2+ entry through a stretch-activated channel, called the transient receptor potential canonical (TRPC) channels, is triggered by eccentric contractions and promotes local depolarization of the SC membrane to initiate the SC activation cascade [17, 18]. However, only a few published studies have compared eccentric exercise with concentric exercise at the same time in human subjects. It is difficult to isolate eccentric and concentric contractions because both types of contractions show an interacting cyclic repeating pattern during normal human movements.
In the study reported here, we investigated the time-course of the expression of several mRNAs and of SC number in vastus lateralis (VL) muscles on both sides of the body after step up/down exercise involving mainly concentric contractions with the right VL muscle and mainly eccentric contractions with the left VL muscle.
Materials and methods
Subjects
Sixteen non-trained young adults (9 males; age 22.4 ± 1.1 years, height 169.8 ± 8.7 cm, weight 58.7 ± 10.7 kg) provided informed consent to participate in the study.
The experiment protocol was approved by the ethics committee of Yamaguchi University. The study and analysis protocols conformed to all local and international standards, including the revised 1983 Declaration of Helsinki, on research involving human subjects.
Exercise protocol and muscle sampling
The subjects performed a 30-min step up/down exercise using steps at the pace of 1 step per 1.5 s. The exercise was performed as follows: forward step up with right leg first with left leg providing support, followed by step up with left leg; then backward step down with right leg first with left leg providing support, followed by step down with left leg (Fig. 1). The height of the step for each subject (38–46 cm) was adjusted according to the height of the subject (156–183 cm). Heart rate was measured during the exercise using a portable heart rate monitor system (FT1; Polar Electro Oy, Kempele, Finland). A visual analog scale (VAS) was used to determine the pain level of the VL muscle as follows: each subject was asked to assess the level of muscle pain on a 10-cm horizontal line marked with numbers from zero to ten (with “0” indicating no pain and “10” indicating the severest pain) at four time periods (before the exercise and at 5 min and 2 and 5 days post-exercise).
Electromyography (EMG) and motion analysis during the step up/down exercise. Surface EMG activity of the vastus lateralis (VL) muscle and angle in the knee joint of the sustained leg on the step were recorded simultaneously using a motion analysis system. Concentric and eccentric contraction phases were identified by changes in EMG amplitude and angle
A needle biopsy was performed before the exercise (Pre) and at 2 and 5 days after the exercise to obtain a muscle sample from the VL muscle. During this procedure, the skin was first anaesthetized with 1 % Xylocaine (AstraZeneca, London, UK) and then muscle samples (approx. 15 mg wet weight) were biopsied from a point of 20 mm away from the other two points at the mid-portion of both sides of the VL muscles. These samples were immediately frozen in liquid nitrogen and stored at −80 °C until analysis.
Knee joint angles and EMG
To measure the changes in the knee joint angle, we placed reflective markers in the greater trochanter, lateral epicondyle of the femur and malleolus lateralis. The EMG activity of the VL muscle and joint angle change in the knee joint were recorded simultaneously using a motion analysis system (1200 Hz in EMG and 120 Hz in video; Fig. 1) (CAPTUREEX; Library, Tokyo, Japan). Surface EMG signals were recorded from the VL muscle using electrodes with a 20-mm inter-electrode distance. The EMG signals were amplified using an EMG amplifier (amplification unit 4124; NEC-Sanei, Tokyo, Japan) and then the analog EMG signals were converted to digital signals and transmitted to a personal computer. Concentric and eccentric contraction phases were identified by changes in the EMG pattern and joint angle using an motion analysis system (Wave Disp; Library, Tokyo, Japan), and the total integrated EMG of each contraction phase was calculated. Concentric and eccentric contractions were defined as “an increasing joint angle with appearance of EMG” and “a decreasing joint angle with appearance of EMG”, respectively.
Immunohistochemistry analysis
The muscle fiber type population was determined using methods described previously [9, 19]. Serial 7-μm-thick cross-sections of the muscle were obtained on a cryostat at −20 °C (model CM510; Leica Microsystems, Wetzlar, Germany). The sections were warmed to room temperature (RT) and then preincubated with 1 % normal goat serum (EMD Millipore, Billerica, MA) in 0.1 μmol/l phosphate buffered saline (PBS; pH 7.6) at RT for 10 min. The primary monoclonal antibody, fast myosin (1:2000; Sigma, St. Louis, MO), which specifically reacts with the myosin heavy chain (MHC) IIa and IIx, was then applied, followed by SC-71 (1:1000; Developmental Studies Hybridoma Bank, Iowa City, IA), which specifically reacts with MHC-IIa. The sections were incubated with these two primary antibodies at 37 °C for 3 h, then washed with PBS and incubated with the secondary antibody [goat anti-mouse (IgG)] conjugated with horseradish peroxidase (HRP) (Bio-Rad, Hercules, CA) for 3 h at 37 °C for 3 h, and finally washed once again with PBS. Diaminobenzidine tetrahydrochloride was used as a chromogen to the localized HRP.
Images of the stained muscle fibers were recorded with a photomicroscopic (E600; Nikon Corp., Tokyo Japan) image-processing system (DS-U1; Nikon Corp.). The fibers were classified as type I, IIa, or IIx fibers based on the immunohistochemical staining properties (Fig. 2), and the population percentage and cross-sectional area of each muscle fiber type were calculated from at least 200 muscle fibers.
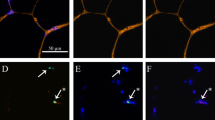
Typical photomicrographs of serial transverse sections of VL muscle. Two sections were stained with monoclonal antibodies against fast myosin (a) and myosin heavy chain (MHC)-IIa (SC-71) (b). Muscle fibers were classified into type I (fibers unstained in both a and b), IIa (stained fibers in all sections), and IIx (stained fibers in a, but unstained in b). c Triple-immunofluorescent stains for laminin, paired box transcription factor 7 (Pax7), and nuclei. White arrows indicate satellite cells (Pax7 + nuclei)
Identification of SC
A serial section from each biopsy was fixed in 2 % paraformaldehyde in 0.1 mol/l PBS at 4 °C for 10 min and washed with PBS. These sections were preincubated with a blocking solution containing 10 % normal goat serum (EMD Millipore) and 2 % bovine serum albumin (BSA; Sigma) in PBS for 30 min. After preincubation, each section was incubated at RT for 3 h with the primary antibodies, a mouse anti-Pax7 (1:1000; Developmental Studies Hybridoma Bank) and rabbit anti-laminin (1:1000; Sigma) that was diluted in 2 % BSA in PBS. This anti-Pax7 is the most widely used antibody for the identification of muscle SCs in some species [20–24]. The section was washed with PBS and treated with the appropriate secondary antibodies—Cy3-conjugated Affini Pure goat anti-mouse IgG (1:1000; Jackson ImmunoResearch, West Grove, Pa) and Alexa Fluoer 488 goat anti-rabbit IgG (1:1000; Molecular Probes, Eugene, OR)—diluted in 2 % BSA in PBS. After incubating at RT for 2 h with the secondary antibodies, the sections were washed with PBS and stained at RT for 5 min with 4,6-diamino-2-phenyl-indole (Molecular Probes) diluted in 2 % BSA in PBS.
Microphotoscopic images of anti-Pax7, anti-laminin, and DAPI were merged using image processing software and used to quantify SCs (Fig. 2c). SCs were identified at the periphery of each fiber beneath the basal lamina and stained for both DAPI and Pax7. The number of SCs per fiber was calculated separately for each of the three fiber types.
Real time reverse transcription-PCR for mRNA
Each muscle sample was analyzed for the expression of several mRNAs using a real time reverse transcription-PCR system. Total RNA was extracted with TORIZOL reagent (Invitrogen, Carlsbad, CA), and the purity and yield of the total RNA extracted was determined by absorbance of aliquots at 260 and 280 nm. Total RNA was then treated with TURBO DNase (Ambion–Life Technologies, Austin, TX) at 37 °C for 30 min to remove genomic DNA from the samples. Samples of DNase-treated RNA (0.5 μg) were used to first-strand cDNA with an Exscript RT Reagent kit (TaKaRa Bio, Otsu, Japan). Thereafter, the cDNA products were analyzed using a Real Time PCR system with the SYBR Green PCR Master Mix protocol in the StepOne™ Real Time PCR System (Applied Biosystems, Foster City, CA).
The sequences of the specific primers used in this study are summarized in Table 1. Each primer was designed using Primer Express software (v3.0; Applied Biosystems), and the oligonucleotides were purchased from FASMAC Co. Ltd (Kanagawa, Japan).
Data analysis
Level of muscle pain, integrated EMG pattern, and number of SCs per muscle fiber were analyzed with one-way analysis of variance followed by the t test with Bonferroni adjustment. The expression of each mRNA at 2 and 5 days after exercise was presented as a relative value for before the exercise (Pre), and these values were compared using the Mann–Whitney U test. In all cases, data were reported as the mean ± standard error, and statistical significance was set at P < 0.05.
Results
Changes in EMG activity and joint angle during exercise
The surface EMG activity, joint angle change in the knee, and total integrated EMG activity during the first set step up/down exercise are shown in Fig. 1. The most effective exercise patterns to induce mainly eccentric contraction in the left leg and mainly concentric contraction in the right leg were as follows: forward step up with right leg, support provided by left leg, and backward step with right leg, with support provided by left leg. In all subjects, the mean value of total integrated EMG activity of the left VL muscle was 70.1 ± 17.4 %, when integrated EMG activity in the right VL muscle was 100 % (Fig. 3a). The percentage of integrated EMG in the eccentric contraction phase relative to total EMG activity was 33.1 ± 15.1 and 73.3 ± 7.7 % in the right and left VL muscles, respectively. The percentage of integrated EMG in the concentric contraction phase relative to total EMG activity was 66.9 ± 15.1 % and 26.7 ± 7.7 % in the right and left VL muscles, respectively.
a Percentage of integrated EMG in eccentric phase (gray) and concentric phase (white) in right and left VL muscles. Data are presented as the relative change against the total integrated EMG activity of the right VL muscle. †Significant difference vs. total integrated EMG in the right VL, *significant difference vs. integrated EMG in each contraction phase of the right VL muscle. b Assessment of muscle pain in VL muscles by the visual analog scale where “0” and “10” indicate no pain and severest pain, respectively. Muscle pain in VL muscles on both sides was evaluated before exercise (Pre), 5 min after exercise (Post), and 2 (2d) and 5 (5d) days after exercise. *Significant difference vs. Pre in each side leg, †significant difference vs. 2 days in right leg. Values are the mean ± standard error (SE)
Exercise intensity and muscle pain
The mean heart rate during the last 1 min of exercise was 138.9 ± 3.9 bpm. As shown in Fig. 3b, muscle pain in VL increased immediately after exercise (left 1.2 ± 0.6, right 0.6 ± 0.2) and peaked at 2 days (left 6.7 ± 0.8, right 2.5 ± 0.7) and then began to decrease by 5 days after exercise (left 1.3 ± 0.6, right 0.7 ± 0.5). Subjects had greater muscle pain in the left VL muscle than in the right VL muscle at 2 days after exercise.
Changes in the number of SCs
Changes in the numbers of SCs of the three fiber types are shown in Fig. 4. The number of SCs per type IIa fiber significantly increased at 2 and 5 days after exercise in the left VL muscle (0.28 ± 0.04 at Pre, 0.36 ± 0.06 at 2 days, 0.42 ± 0.07 at 5 days). In comparison, the number of SCs per type IIa fiber significantly increased only at 5 days after exercise in the right VL muscle (0.27 ± 0.05 at Pre, 0.41 ± 0.07 at 5 days). The number of SCs per type I fiber and type IIx fiber showed a tendency to increase in the VL muscles of both sides. The histological studies did not reveal any significant sex-related differences.
Change in the number of satellite cells (SCs) in the VL muscles on both sides. Immunohistochemical analysis was performed for biopsy samples obtained before exercise (Pre) and at 2 (2d) and 5 (5d) days after exercise from VL muscles on both sides. *Significant difference from the Pre VL on both sides for each fiber type (P < 0.05)
Change in the expression of mRNA
The expression of MyoD mRNA was significantly increased in the left VL muscle at 2 days after exercise (1.43 ± 0.17) but not in the right VL muscle (Fig. 5). The expression of both Pax7 and myogenin mRNAs in the left VL muscle tended to increase after exercise. As shown in Fig. 6, the expression of HGF mRNA had significantly increased at 2 and 5 days after exercise in the left VL muscle (3.92 ± 1.33 and 3.36 ± 1.19, respectively) and at 2 days in the right VL muscle (3.29 ± 1.25). The expression of c-Met mRNA and TRPC1 increased at 2 days after exercise in the left VL muscle (1.45 ± 0.11 and 4.02 ± 2.74, respectively), but not in the right VL muscle. The expression of TRPC6 mRNA showed a tendency to increase at 2 and 5 days after exercise in the left VL muscle (2.92 ± 1.57 and 3.91 ± 2.82, respectively), but not in the right VL muscle. The expression of myostatin mRNA had significantly increased at 2 and 5 days after exercise in the left VL muscle (1.40 ± 0.22 and 1.65 ± 0.17, respectively), but not in the right VL muscle. The expression of interleukin-6, a typical inflammatory marker, mRNA tended to increase at 5 days after exercise in the left VL muscle (3.45 ± 0.18). There were no significant sex-related differences in mRNA expression.
Change in the expression of mRNA of myogenic regulation factors in VL muscles of both sides. Expression of paired box transcription factor 7 (Pax7; a), myogenic determination factor 1 (MyoD; b), myogenin (c) mRNA at 2 (2d) and 5 (5d) days after exercise were calculated as a fold change from the Pre values in VL muscles of both sides. *Significant difference vs. Pre (P < 0.05)
Changes in mRNA expression in VL muscles of both sides. Expression of hepatocyte growth factor (HGF; a), c-Met (b), transient receptor potential canonical 1 (TRPC1; c), TRPC6 (d), myostatin (e), and interleukin-6 (IL-6; f) mRNA at 2 (2d) and 5 (5d) days after exercise. Values were calculated as fold change from the Pre values in VL muscles of both sides. *Significant difference vs. Pre (P < 0.05)
Discussion
Eccentric contractions in VL muscles during Step up/down exercise
In this study, the mean value of integrated EMG in the left VL muscle was significantly lower than that in the right VL muscle, but the left VL muscle showed more severe muscle pain than the right. In previous studies, eccentric contraction was in particular likely to cause muscle damage characterized by immediate weakness and a more slowly develo** stiffness, soreness, and swelling [25–29]. Eccentric contractions cause sarcomere inhomogeneity, as well as the loss of the normal continuity of Z lines [25, 26]. They also increase membrane permeability, which allows soluble cytosolic proteins, such as creatine kinase, to leak out of the muscle and into the plasma [27], whereas extracellular proteins, such as albumin and fibronectin, can be detected inside the muscle [28, 29]. Although we did not find obvious histological damage, such as centrally nucleated fibers and destruction of basement membrane, the expression of IL-6 mRNA tended to increase at 5 days after exercise in the left VL muscle, possibly suggesting micro-disorders of the muscle fibers.
Proliferation of SCs following exercise
A significant increase in the number of SCs per IIa fiber type was observed at 2 and 5 days after exercise in the left VL muscle and only at 5 days after exercise in the right VL muscle. These results suggest that eccentric contraction may impact the activation and proliferation of SCs more than concentric contraction. In addition, the expression of MyoD mRNA increased at 2 and 5 days after exercise in the left VL muscle, which are in agreement with the results of the immunohistochemical analysis. In contrast, the expression of MyoD mRNA in the right VL muscle did not show any significant change. The expression of Pax7 and myogenin mRNA tended to increase in the left VL muscle after exercise. Previous studies measuring myogenic regulation factor expression following eccentric exercise have reported an increase in MyoD at 4 h post-exercise, which returned to the baseline level at 24 h post-exercise [16]. Other studies have reported an increase in myogenin expression at 1 week after exercise [9]. Although we did not investigate mRNA expression at 1 week after exercise, the expression of myogenin mRNA may be increased to regulate terminal differentiation after 7 days.
Many studies have demonstrated that growth factors, such as HGF, are associated with the activation and proliferation of myoblasts [30–32]. Quiescent and activated SCs have been shown in vitro and in vivo to express the c-Met receptor that mediates the intracellular signaling response of HGF [31, 33]. A model has been proposed in which HGF pooled in the extracellular domain of skeletal muscle fibers is released rapidly by matrix metalloproteinase activity caused by the production of nitric oxide (NO) radicals following the mechanical stretching of muscle tissue [34]. HGF subsequently binds to the c-Met receptor to generate a signal for SC activation [34]. In the present study, the increase in both HGF and c-Met mRNA expression in the left VL muscle suggests that eccentric contraction enhances the expression of these mRNAs more than concentric exercise. Although the expressions of HGF and C-Met RNAs did significantly differ from each other, the time-courses of their up- and downregulation were very similar. This result may indicate that a pathway of HGF/c-Met signaling plays a very important role after the eccentric contraction in human muscles. This notion is good agreement with the findings of studies which showed that released HGF induces c-Met expression as an immediate-early gene and thus also enhances HGF/c-Met signaling in the SC activation process [18, 35]. The observed increase in the expression of these mRNAs in the left VL muscle might occur simultaneously with the change in MyoD mRNA expression. These results suggest that SC activation and proliferation through HGF/c-Met signaling may be promoted more by eccentric exercise than by concentric exercise.
In SC cultures, cations may flow in through TRPC1, which is a stretch-activated cation channel in muscle fibers and SCs, thereby promoting the local depolarization of SCs membranes to initiate the SC activation cascade [18]. In this study, the expression of TRPC1 mRNA significantly increased at 2 days after exercise in the left VL muscle. Furthermore, although the change was not significant, the expression of TRPC6 mRNA tended to increase at 2 and 5 days after exercise in the left VL muscle. These observations may suggest that the activation of SCs by stretch is a cascade of sequential events, including an influx of cations and depolarization of cell membranes, an influx of calcium ions, the binding of calcium ions to calmodulin, NO radical production by constitutive NO synthases, activation of matrix metalloproteinases, HGF release from the matrix, and presentation of HGF to the signaling receptor c-Met [18, 34, 36]. In addition, the flow of calcium ions through TRPC1, which is related to the activation of calpain, causes proteolysis of muscle cytoskeletal proteins [18]. Activation of calpain may explain the results indicating greater muscle pain in the left leg. In their study, Hara et al. [18] noted the importance of another type of receptor, TRPC5, which they described in cultured cells. Unfortunately, although we were unable to detect the expression of TRPC5 mRNA in this study, TRPC1 and TRPC6 seem to be reasonable candidates for the mechanical-stretch channel in the satellite cell activation pathway after eccentric contraction of human anti-gravity muscles.
Myostatin is a member of the transforming growth factor-β superfamily and a strong negative regulator of skeletal muscle mass [37–39]. Recombinant myostatin has been shown to reversibly inhibit C2C12 myoblast proliferation by arresting cells in the G1 and G2/M stages of the cell cycle [40, 41]. In many studies, downregulated myostatin mRNA expression has been observed in response to exercise training [42–44]. An unexpected finding of our study was that the expression of myostatin mRNA increased in the left VL muscle at 2 and 5 days after exercise. In an earlier study, Yamada et al. [45] reported high concentrations of HGF with upregulated myostatin mRNA expression, as well as enhanced expression and secreation of myostatin protein in vitro in SC cultures. These authors hypothesized that HGF may play dual roles in SC activation, as follows. The initiation of SC activation indeed requires HGF, but a low amount of extracellular HGF may be sufficient for the rapid activation of SCs through the growth factor ligand/high-affinity receptor c-Met system. During muscle regeneration and hypertrophy, HGF synthesis is initiated in the SCs of the other cells, and when extracellular HGF concentrations reach a sufficiently high threshold to enable association with an unidentified low-affinity receptor, SCs would start expressing myostatin. Our results of relative mRNA expressions supports the notion that the expression of myostatin increases in order to control any excessive increase in SCs following eccentric exercise.
The differences in the time-course of SC activation between eccentric and concentric contractions were investigated in the VL muscle after step up/down exercise. The number of SCs had significantly increased in type IIa fibers of the left VL, involving mainly eccentric contractions. The expression of HGF, TRPC1, and MyoD mRNAs had significantly increased in the left VL at 2 days. These results indicate that eccentric contractions can effectively activate SC proliferation after exercise. Understanding these mechanisms of SC activation related to eccentric exercise provides fundamental information applicable for use in training methods for human antigravity muscles.
References
Molinari F, Knaflitz M, Bonato P (2006) Electrical manifestations of muscle fatigue during concentric and eccentric isokinetic knee flexion-extension movements. IEEE Trans Biomed Eng 53:1309–1316
Okamoto T, Masuhara M, Ikuta K (2006) Cardiovascular responses induced during high-intensity eccentric and concentric isokinetic muscle contraction in healthy young adults. Clin Physiol Funct Imaging 26:39–44
Roig M, O’Brien K, Kirk G, Murray R, McKinnon P et al (2009) The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. Br J Sports Med 43:556–568
Vallejo AF, Schroeder ET, Zheng L, Jensky NE, Sattler FR (2006) Cardiopulmonary responses to eccentric and concentric resistance exercise in older adults. Age Ageing 35:291–297
Enoka RM (1996) Eccentric contractions require unique activation strategies by the nervous system. J Appl Physiol 81:2339–2346
Lieber RL, Friden J (1988) Selective damage of fast glycolytic muscle fibers with eccentric contraction of the rabbit tibialis anterior. Acta Physiol Scand 133:587–588
Nardone A, Romano C, Schieppati M (1989) Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol 409:451–471
Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493–495
Kawai M, Aida H, Hiraga A, Miyata H (2013) Muscle satellite cells are activated after exercise to exhaustion in thoroughbred horses. Equine Vet J 45:512–517
Chargé SBP, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Kuang S, Rudnicki MA (2008) The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med 14:82–91
McKinnell IW, Ishibashi J, Le GF, Punch VG, Addicks GC, Greenblatt JF, Dilworth FJ, Rudnicki MA (2008) Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol 10:77–84
Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne GS (1999) In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci 112:2895–2901
Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA (1999) Reduced differentiation potential of primary MyoD–/–myogenic cells derived from adult skeletal muscle. J Cell Biol 144:631–643
McKay BR, O’Reilly CE, Phillips SM, Tarnopolsky MA, Parise G (2008) Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. J Physiol 586:5549–5560
O’Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G (2008) Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve 38:1434–1442
Zhang BT, Whitehead NP, Gervasio OL, Reardon TF, Vale M, Fatkin D, Dietrich A, Yeung EW, Allen DG (2012) Pathways of Ca2+ entry and cytoskeletal damage following eccentric contractions in mouse skeletal muscle. J Appl Physiol 112:2077–2086
Hara M, Tabata K, Suzuki T, Do MQ, Mizunoya W, Nakamura M, Nishimura S, Tabata S, Ikeuchi Y, Sunagawa K, Anderson JE, Allen RE, Tatsumi R (2012) Calcium influx through a possible coupling of cation channels impacts skeletal muscle satellite cell activation in response to mechanical stretch. Am J Physiol Cell Physiol 302:C1741–C1750
Yamano S, Eto D, Kasashima Y, Hiraga A, Sugiura T, Miyata H (2005) Evaluation of developmental changes in the coexpression of myosin heavy chains and metabolic properties of equine skeletal muscle fibers. Am J Vet Res 66:401–405
Imaoka Y, Kawai M, Mukai K, Ohmura H, Takahashi T, Hiraga A, Miyata H (2014) Training and detraining effects on satellite cell response after exhaustive exercise in thoroughbred horses. Jpn J Phys Fit Sports Med 63:177–187
Kurosaka M, Naito H, Ogura Y, Machida S, Katamoto S (2012) Satellite cell pool enhancement in rat plantaris muscle by endurance training depends on intensity rather than duration. Acta Physiol 205:159–166
Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P (2008) Interleukin-6 is an essential regulator of satellite cell- mediated skeletal muscle hypertrophy. Cell Metab 7:33–44
Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA et al (2004) Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 166:347–357
McKay BR, Toth KG, Tarnopolsky MA, Parise G (2010) Satellite cell number and cell cycle kinetics in response to acute myotrauma in humans: immunohistochemistry versus flow cytometry. J Physiol 588:3307–3320
Fridén J, Sjöström M, Ekblom B (1981) A morphological study of delayed muscle soreness. Experientia 37:506–507
Takekura H, Fu**ami N, Nishizawa T, Ogasawara H, Kasuga N (2001) Eccentric exercise-induced morphological changes in the membrane systems involved in excitation-contraction coupling in rat skeletal muscle. J Physiol 533:571–583
Newham DJ, Jones DA, Edwards RH (1986) Plasma creatine kinase changes after eccentric and concentric contractions. Muscle Nerve 9:59–63
Lieber RL, Schmitz MC, Mishra DK, Friden J (1994) Contractile and cellular remodeling in rabbit skeletal muscle after cyclic eccentric contractions. J Appl Physiol 77:1926–1934
McNeil PL, Khakee R (1992) Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol 140:1097–1109
Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR (1997) The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 272:6653–6662
Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE (2002) Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell 13:2909–2918
Ates K, Yang SY, Orrell RW, Sinanan AC, Simons P, Solomon A, Beech S, Goldspink G, Lewis MP (2007) The IGF-I splice variant MGF increases progenitor cells in ALS, dystrophic, and normal muscle. FEBS Lett 581:2727–2732
McKay BR, Toth KG, Tarnopolsky MA, Parise G (2010) Satellite cell number and cell cycle kinetics in response to acute myotrauma in humans: immunohistochemistry versus flow cytometry. J Physiol 588:3307–3320
Tatsumi R (2010) Mechano-biology of skeletal muscle hypertrophy and regeneration: possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim Sci J 81:11–20
Wozniak AC, Anderson JE (2007) Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev Dyn 236:240–250
Tatsumi R, Allen RE (2004) Active hepatocyte growth factor is present in skeletal muscle extracellular matrix. Muscle Nerve 30:654–658
Argilés JM, Orpí M, Busquets S, López-Soriano FJ (2012) Myostatin: more than just a regulator of muscle mass. Drug Discov Today 17:702–709
Lee SJ, McPherron AC (1999) Myostatin and the control of skeletal muscle mass. Curr Opin Genet Dev 9:604–607
Tsuchida K (2008) Targeting myostatin for therapies against muscle-wasting disorders. Curr Opin Drug Discov Devel 11:487–494
Ríos R, Carneiro I, Arce VM, Devesa J (2002) Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol 282:C993–C999
Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R (2000) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243
Costa A, Dalloul H, Hegyesi H, Apor P, Csende Z et al (2007) Impact of repeated bouts of eccentric exercise on myogenic gene expression. Eur J Appl Physiol 101:427–436
Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, Petersson SJ, Schrøder HD, Andersen JL, Aagaard P, Schjerling P, Kjaer M (2013) Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J Physiol 591:3789–3804
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe SW (2006) Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 years) and old (80–89 years) women. J Appl Physiol 101:53–59
Yamada M, Tatsumi R, Yamanouchi K, Hosoyama T, Shiratsuchi S, Sato A, Mizunoya W, Ikeuchi Y, Furuse M, Allen RE (2010) High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol Cell Physiol 298:C465–C476
Acknowledgments
This work was supported in part by a grant from The Japanese Ministry of Education, Science and Culture (No. 23650434).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Imaoka, Y., Kawai, M., Mori, F. et al. Effect of eccentric contraction on satellite cell activation in human vastus lateralis muscle. J Physiol Sci 65, 461–469 (2015). https://doi.org/10.1007/s12576-015-0385-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-015-0385-4