Abstract
Introduction
The benefits of osteoporosis therapy are compromised by low adherence, thus requiring a better understanding of its barriers and unmet needs. The objective of this study was to assess reasons for non-adherence with oral bisphosphonates among osteoporotic women.
Methods
A cross-sectional patient survey of women who initiated therapy with risedronate or alendronate between the years 2010 and 2012 were non-adherent [Medication Possession Ratio (MPR) <70%] or switched therapy within the first year. Survey participants were identified using Maccabi Health Services computerized database. Patients who gave informed consent completed a 20-min telephonic survey, assessing reasons for discontinuation or switching, including physician involvement, side effects, administration regimen, perceptions of bone health, and medications’ efficacy.
Results
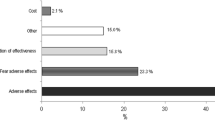
The study population included 493 females (mean age = 66 ± 7) of whom 40% discontinued all anti-osteoporotic therapy (mean MPR = 19%), 9% remained on initial therapy (mean MPR = 47%), and 51% switched therapy (mean MPR = 62%). Family history, fracture history, socioeconomic status, and index drug class and frequency were similar in all groups. The most common reasons for switching or discontinuation of the first-line therapy were gastrointestinal side effects, such as heartburn, acid reflux or other (40.0%), and physician recommendation (26.7%). The major reasons for complete discontinuation of therapy were side effects (26.9%) and physician recommendation (20.0%). Perceived low importance was more commonly mentioned than high cost of medication (14% vs. 3%).
Conclusion
Our findings highlight the importance of low tolerability to non-adherence with osteoporosis therapy and underlines poor patients’ awareness and sub-optimal physicians’ involvement in conveying the importance of this therapy.
Funding
Merck & Co Inc.



Similar content being viewed by others
References
Kanis JA, Melton LR, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–41.
MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148(3):197–213.
Hadji P, Claus V, Ziller V, Intorcia M, Kostev K, Steinle T. GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int. 2012;23(1):223–31.
Imaz I, Zegarra P, Gonzalez-Enriquez J, Rubio B, Alcazar R, Amate JM. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010;21(11):1943–51.
Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38(6):922–8.
Rabenda V, Hiligsmann M, Reginster JY. Poor adherence to oral bisphosphonate treatment and its consequences: a review of the evidence. Expert Opin Pharmacother. 2009;10(14):2303–15.
Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Managed Care Pharm JMCP. 2009;15(9):728–40.
Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81(8):1013–22.
Rabenda V, Mertens R, Fabri V, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19(6):811–8.
Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122(2 Suppl):S3–13.
Ross S, Samuels E, Gairy K, Iqbal S, Badamgarav E, Siris E. A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value Health. 2011;14(4):571–81.
Hiligsmann M, Rabenda V, Bruyere O, Reginster JY. The clinical and economic burden of non-adherence with oral bisphosphonates in osteoporotic patients. Health Policy. 2010;96(2):170–7.
Mukhtar O, Weinman J, Jackson SH. Intentional non-adherence to medications by older adults. Drugs Aging. 2014;31(3):149–57.
Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24.
Reynolds K, Viswanathan HN, Muntner P, et al. Validation of the Osteoporosis-Specific Morisky Medication Adherence Scale in long-term users of bisphosphonates. Qual Life Res. 2014;23(7):2109–20.
Flood EM, Beusterien KM, Green H, et al. Psychometric evaluation of the Osteoporosis Patient Treatment Satisfaction Questionnaire (OPSAT-Q), a novel measure to assess satisfaction with bisphosphonate treatment in postmenopausal women. Health Qual Life Outcomes. 2006;4:42.
Chandler JM, Martin AR, Girman C, et al. Reliability of an Osteoporosis-Targeted Quality of Life Survey Instrument for use in the community: OPTQoL. Osteoporos Int. 1998;8(2):127–35.
Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003;14(3):259–62.
Turbi C, Herrero-Beaumont G, Acebes JC, et al. Compliance and satisfaction with raloxifene versus alendronate for the treatment of postmenopausal osteoporosis in clinical practice: an open-label, prospective, nonrandomized, observational study. Clin Ther. 2004;26(2):245–56.
McHorney CA, Schousboe JT, Cline RR, Weiss TW. The impact of osteoporosis medication beliefs and side-effect experiences on non-adherence to oral bisphosphonates. Curr Med Res Opin. 2007;23(12):3137–52.
Woo C, Gao G, Wade S, Hochberg MC. Gastrointestinal side effects in postmenopausal women using osteoporosis therapy: 1-year findings in the POSSIBLE US study. Curr Med Res Opin. 2010;26(4):1003–9.
Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–94.
Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1–23.
Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–91.
Silverman SL, Landesberg R. Osteonecrosis of the jaw and the role of bisphosphonates: a critical review. Am J Med. 2009;122(2 Suppl):S33–45.
Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11(7):418–28.
Hiligsmann M, Salas M, Hughes DA, et al. Interventions to improve osteoporosis medication adherence and persistence: a systematic review and literature appraisal by the ISPOR Medication Adherence and Persistence Special Interest Group. Osteoporos Int. 2013;24(12):2907–18.
Nielsen D, Ryg J, Nielsen W, Knold B, Nissen N, Brixen K. Patient education in groups increases knowledge of osteoporosis and adherence to treatment: a two-year randomized controlled trial. Patient Educ Couns. 2010;81(2):155–60.
Cook PF, Emiliozzi S, McCabe MM. Telephone counseling to improve osteoporosis treatment adherence: an effectiveness study in community practice settings. Am J Med Qual. 2007;22(6):445–56.
Solomon DH, Katz JN, Finkelstein JS, et al. Osteoporosis improvement: a large-scale randomized controlled trial of patient and primary care physician education. J Bone Miner Res. 2007;22(11):1808–15.
Shu AD, Stedman MR, Polinski JM, et al. Adherence to osteoporosis medications after patient and physician brief education: post hoc analysis of a randomized controlled trial. Am J Manag Care. 2009;15(7):417–24.
Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(3):1117–23.
Delmas PD, Vrijens B, Eastell R, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007;92(4):1296–304.
Pickney CS, Arnason JA. Correlation between patient recall of bone densitometry results and subsequent treatment adherence. Osteoporos Int. 2005;16(9):1156–60.
Silverman SL, Nasser K, Nattrass S, Drinkwater B. Impact of bone turnover markers and/or educational information on persistence to oral bisphosphonate therapy: a community setting-based trial. Osteoporos Int. 2012;23(3):1069–74.
Compston J. Monitoring osteoporosis treatment. Best Pract Res Clin Rheumatol. 2009;23(6):781–8.
Cooper A. Compliance with treatment for osteoporosis. Lancet. 2006;368(9548):1648.
Kendler DL, McClung MR, Freemantle N, et al. Adherence, preference, and satisfaction of postmenopausal women taking denosumab or alendronate. Osteoporos Int. 2011;22(6):1725–35.
Freemantle N, Satram-Hoang S, Tang ET, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23(1):317–26.
Cotte FE, Fardellone P, Mercier F, Gaudin AF, Roux C. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010;21(1):145–55.
Hill DA, Cacciatore M, Lamvu GM. Electronic prescribing influence on calcium supplementation: a randomized controlled trial. Am J Obstetr Gynecol. 2010;202(3):236.
Alamri SH, Kennedy CC, Marr S, Lohfeld L, Skidmore CJ, Papaioannou A. Strategies to overcome barriers to implementing osteoporosis and fracture prevention guidelines in long-term care: a qualitative analysis of action plans suggested by front line staff in Ontario, Canada. BMC Geriatr. 2015;15:94.
Reginster JY, Neuprez A, Lecart MP, et al. Osteoporosis and personalized medicine. Rev Med Liege. 2015;70(5–6):321–4.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Merck and co. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Inbal Goldshtein, Vanessa Rouach, Naama Shamir-Stein, and Gabriel Chodick have nothing to disclose. **gbo Yu was a Merck employee at the time of the study.
Compliance with Ethics Guidelines
The study protocol was approved by the MHS and Assuta Health Systems ethical review board. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Inbal Goldshtein and Vanessa Rouach contributed equally to this work.
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/B9D4F06065B72F60.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goldshtein, I., Rouach, V., Shamir-Stein, N. et al. Role of Side Effects, Physician Involvement, and Patient Perception in Non-Adherence with Oral Bisphosphonates. Adv Ther 33, 1374–1384 (2016). https://doi.org/10.1007/s12325-016-0360-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0360-3