Abstract
Background
The aim of this large nationwide study was to validate two novel composite treatment scores that address guideline-concordant locoregional and systemic breast cancer care. We examined the relationship between these two scores and their association with survival.
Methods
Women with Stage I–III unilateral breast cancer were identified within the National Cancer Database. For each woman, a locoregional and a systemic treatment score (0, 1, 2) was assigned based on receipt of guideline-concordant care. Multivariable Cox regression models evaluated the association between the scores and survival.
Results
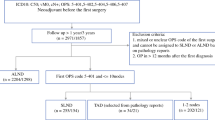
623,756 women were treated at 1,221 different American College of Surgeons Commission on Cancer (CoC) facilities. Overall, 86% had a locoregional treatment score of 2 (most guideline-concordant), 75% had a systemic treatment score of 2, and 72% had both scores of 2. Median follow-up was 4.5 years. Compared to women with a locoregional treatment score of 2, those with a score of 1 or 0 had a 1.7-fold and 2.0-fold adjusted greater risk of death. Compared to women with a systemic treatment score of 2, those with a score of 1 or 0 had a 1.5-fold and 2.1-fold adjusted greater risk of death. Risk-adjusted 5-year overall survival was 91.6% when both scores were 2 compared to 73.4% when both scores were 0.
Conclusions
In this large national study of CoC facilities, two composite scores capturing guideline-concordant breast cancer care had independent and combined robust effects on survival. These clinically constructed novel scores are promising tools for health services research and quality-of-care studies.



Similar content being viewed by others
References
Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32:1507–15. https://doi.org/10.1200/JCO.2013.53.3935.
Early Breast Cancer Trialists’ Collaborative Group, McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–35. https://doi.org/10.1016/S0140-6736(14)60488-8.
Early Breast Cancer Trialists’ Collaborative Group, Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–16. https://doi.org/10.1016/S0140-6736(11)61629-2.
Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. https://doi.org/10.1016/S0140-6736(05)66544-0.
Early Breast Cancer Trialists’ Collaborative Group, Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–44. https://doi.org/10.1016/S0140-6736(11)61625-5.
Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52. https://doi.org/10.1016/S0140-6736(15)61074-1.
National Quality Forum. News and releases. Endorsement summaries. Cancer. https://www.qualityforum.org/News_And_Resources/Endorsement_Summaries/Endorsement_Summaries.aspx. Accessed 17 July 2020.
American College of Surgeons. Quality programs. Cancer. National Cancer Data Base. CoC Quality of Care Measures. 2019. https://www.facs.org/quality%20programs/cancer/ncdb/qualitymeasures. Accessed 17 July 2020.
Yen TW, Pezzin LE, Li J, et al. Effect of hospital volume on processes of breast cancer care: A National Cancer Data Base study. Cancer. 2017;123(6):957–66. https://doi.org/10.1002/cncr.30413.
American College of Surgeons. Quality programs. Cancer. National Cancer Database https://www.facs.org/quality-programs/cancer/ncdb. Accessed 17 July 2020.
Recht A, Edge SB, Solin LJ, Robinson DS, Estabrook A, Fine RE, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–69. https://doi.org/10.1200/JCO.2001.19.5.1539.
ASCO. American Society of Clinical Oncology. Research & guidelines. Guidelines, tools, & resources. Breast Cancer. https://www.asco.org/research-guidelines/quality-guidelines/guidelines/breast-cancer. Accessed 17 July 2020.
Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–7. https://doi.org/10.1001/jama.286.12.1494.
Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688–97. https://doi.org/10.1200/JCO.2001.19.6.1688.
Macmillan RD, Purushotham AD, Mallon E, Love JG, George WD. Tumour bed positivity predicts outcome after breast-conserving surgery. Br J Surg. 1997;84:1559–62.
DiBiase SJ, Komarnicky LT, Schwartz GF, **e Y, Mansfield CM. The number of positive margins influences the outcome of women treated with breast preservation for early stage breast carcinoma. Cancer. 1998;82:2212–20.
Maishman T, Cutress RI, Hernandez A, Gerty S, Copson ER, Durcan L, et al. Local recurrence and breast oncological surgery in young women with breast cancer: The POSH Observational Cohort Study. Ann Surg. 2017;266:165–72. https://doi.org/10.1097/SLA.0000000000001930.
Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2014;21:717–30. https://doi.org/10.1245/s10434-014-3480-5.
Botteri E, Bagnardi V, Rotmensz N, Gentilini O, Disalvatore D, Bazolli B, et al. Analysis of local and regional recurrences in breast cancer after conservative surgery. Ann Oncol. 2010;21:723–8. https://doi.org/10.1093/annonc/mdp386.
Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–73. https://doi.org/10.1200/JCO.2008.19.8424.
Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE Jr, Jeong JH, Tan-Chiu E, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–37. https://doi.org/10.1200/JCO.2005.04.3273.
Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer. 2006;106:35–41. https://doi.org/10.1002/cncr.21551.
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. https://doi.org/10.1016/S0140-6736(05)67887-7.
Vicini FA, Kestin L, Huang R, Martinez A. Does local recurrence affect the rate of distant metastases and survival in patients with early-stage breast carcinoma treated with breast-conserving therapy? Cancer. 2003;97:910–9. https://doi.org/10.1002/cncr.11143.
Fortin A, Larochelle M, Laverdiere J, Lavertu S, Tremblay D. Local failure is responsible for the decrease in survival for patients with breast cancer treated with conservative surgery and postoperative radiotherapy. J Clin Oncol. 1999;17:101–9. https://doi.org/10.1200/JCO.1999.17.1.101.
Veronesi U, Marubini E, Del Vecchio M, Manzari A, Andreola S, Greco M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst. 1995;87:19–27. https://doi.org/10.1093/jnci/87.1.19.
Whelan T, Clark R, Roberts R, Levine M, Foster G. Ipsilateral breast tumor recurrence postlumpectomy is predictive of subsequent mortality: results from a randomized trial. Investigators of the Ontario Clinical Oncology Group. Int J Radiat Oncol Biol Phys. 1994;30:11–6. https://doi.org/10.1016/0360-3016(94)90513-4.
Fisher B, Anderson S, Fisher ER, Redmond C, Wickerham DL, Wolmark N, et al. Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet. 1991;338:327–31. https://doi.org/10.1016/0140-6736(91)90475-5.
Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21:261–6. https://doi.org/10.1016/j.breast.2011.12.002.
Kantor O, Wang CH, Yao K. Regional variation in performance for commission on cancer breast quality measures and impact on overall survival. Ann Surg Oncol. 2018;25:3069–75. https://doi.org/10.1245/s10434-018-6592-5.
American College of Surgeons. National Cancer Data Base participant use data file (PUF) data dictionary. Version: PUF 2014, p.187. https://www.facs.org/~/media/files/quality%20programs/cancer/ncdb/puf%20data%20dictionary%20version%20puf%202014.ashx. Accessed December 4, 2020.
Funding
No source of funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors (Yen, Garacci, Laud, Pezzin, Nattinger) declare no conflicts of interest.
Research involving human participants and ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this retrospective study, formal informed consent is not required. This de-identified database study (PRO00030472) was determined that it does not meet criteria for human subject research at 45 CFR 46.102 by the Medical College of Wisconsin/Froedtert Hospital Institutional Review Board #5.
Informed consent
A waiver of the informed consent process was granted by the institutional IRB as this is a retrospective, de-identified database study that contains no Protected Health Information (PHI) and does not involve interactions or interventions with living human beings. The waiver of informed consent does not adversely affect the rights and welfare of the subjects and it would not be practical to conduct the research without a waiver.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yen, T.W.F., Garacci, Z., Laud, P.W. et al. Guideline-concordant treatment predicts survival: a National Cancer Database validation study of novel composite locoregional and systemic treatment scores among women with early stage breast cancer. Breast Cancer 28, 698–709 (2021). https://doi.org/10.1007/s12282-020-01206-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-020-01206-9