Abstract
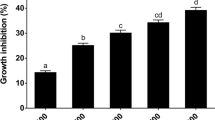
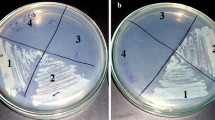
Caulobacter crescentus is an aquatic Gram-negative bacterium that lives in nutrient-poor environments. Like several other aquatic and phytopathogenic bacteria, Caulobacter cells have a relatively large number of genes predicted to encode TonB-dependent receptors (TBDRs). TBDRs transport nutrients across the outer membrane using energy from the proton motive force. We identified one TBDR gene, sucA, which is situated within a cluster of genes predicted to encode a lacI-family transcription factor (sucR), amylosucrase (sucB), fructokinase (sucC), and an inner membrane transporter (sucD). Given its genomic neighborhood, we proposed that sucA encodes a transporter for sucrose. Using RT-qPCR, we determined that expression of sucABCD is strongly induced by sucrose in the media and repressed by the transcription factor, SucR. Furthermore, cells with a deletion of sucA have a reduced uptake of sucrose. Although cells with a non-polar deletion of sucA can grow with sucrose as the sole carbon source, cells with a polar deletion that eliminates expression of sucABCD cannot grow with sucrose as the sole carbon source. These results show that the suc locus is essential for sucrose utilization while SucA functions as one method of sucrose uptake in Caulobacter crescentus. This work sheds light on a new carbohydrate utilization locus in Caulobacter crescentus.
Similar content being viewed by others
References
Blanvillain, S., Meyer, D., Boulanger, A., Lautier, M., Guynet, C., Denance, N., Vasse, J., Lauber, E., and Arlat, M. 2007. Plant carbohydrate scavenging through tonB-dependent receptors: a feature shared by phytopathogenic and aquatic bacteria. PLoS One 2, e224.
Boulanger, A., Zischek, C., Lautier, M., Jamet, S., Rival, P., Carrère, S., Arlat, M., and Lauber, E. 2014. The plant pathogen Xanthomonas campestris pv. campestris exploits N-acetylglucosamine during infection. MBio 5, e01527–01514.
Braun, V. 2006. Energy transfer between biological membranes. ACS Chem. Biol. 1, 352–354.
Chow, V., Shantharaj, D., Guo, Y., Nong, G., Minsavage, G.V., Jones, J.B., and Preston, J.F. 2015. Xylan utilization regulon in Xanthomonas citri pv. citri strain 306: gene expression and utilization of oligoxylosides. Appl. Environ. Microbiol. 81, 2163–2172.
Curtis, P.D. and Brun, Y.V. 2010. Getting in the loop: Regulation of development in Caulobacter crescentus. Microbiol. Mol. Biol. Rev. 74, 13–41.
de Boer, H.A., Comstock, L.J., and Vasser, M. 1983. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc. Natl. Acad. Sci. USA 80, 21–25.
Dejean, G., Blanvillain-Baufume, S., Boulanger, A., Darrasse, A., Duge de Bernonville, T., Girard, A.L., Carrere, S., Jamet, S., Zischek, C., Lautier, M., et al. 2013. The xylan utilization system of the plant pathogen Xanthomonas campestris pv campestris controls epiphytic life and reveals common features with oligotrophic bacteria and animal gut symbionts. New Phytol. 198, 899–915.
Eisenbeis, S., Lohmiller, S., Valdebenito, M., Leicht, S., and Braun, V. 2008. NagA-dependent uptake of N-acetyl-glucosamine and N-acetyl-chitin oligosaccharides across the outer membrane of Caulobacter crescentus. J. Bacteriol. 190, 5230–5238.
Ely, B. 1991. Genetics of Caulobacter crescentus. Methods Enzymol. 204, 372–384.
Evinger, M. and Agabian, N. 1977. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J. Bacteriol. 132, 294–301.
Hottes, A.K., Meewan, M., Yang, D., Arana, N., Romero, P., McAdams, H.H., and Stephens, C. 2004. Transcriptional profiling of Caulobacter crescentus during growth on complex and minimal media. J. Bacteriol. 186, 1448–1461.
Jordan, L.D., Zhou, Y., Smallwood, C.R., Lill, Y., Ritchie, K., Yip, W.T., Newton, S.M., and Klebba, P.E. 2013. Energy-dependent motion of TonB in the Gram-negative bacterial inner membrane. Proc. Natl. Acad. Sci. USA 110, 11553–11558.
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M., and Tanabe, M. 2016. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–462.
Koebnik, R. 2005. TonB-dependent trans-envelope signalling: the exception or the rule? Trends Microbiol. 13, 343–347.
Kohler, C., Lourenço, R.F., Bernhardt, J., Albrecht, D., Schüler, J., Hecker, M., and Gomes, S.L. 2015. A comprehensive genomic, transcriptomic and proteomic analysis of a hyperosmotic stress sensitive α-proteobacterium. BMC Microbiol. 15, 71.
Lasker, K., Schrader, J.M., Men, Y., Marshik, T., Dill, D.L., McAdams, H.H., and Shapiro, L. 2016. CauloBrowser: A systems biology resource for Caulobacter crescentus. Nucleic Acids Res. 44, D640–645.
Lim, H.N., Lee, Y., and Hussein, R. 2011. Fundamental relationship between operon organization and gene expression. Proc. Natl. Acad. Sci. USA 108, 10626–10631.
Lohmiller, S., Hantke, K., Patzer, S.I., and Braun, V. 2008. TonBdependent maltose transport by Caulobacter crescentus. Microbiology 154, 1748–1754.
Martens, E.C., Koropatkin, N.M., Smith, T.J., and Gordon, J.I. 2009. Complex glycan catabolism by the human gut microbiota: The bacteroidetes Sus-like paradigm. J. Biol. Chem. 284, 24673–24677.
Mazzon, R.R., Braz, V.S., da Silva Neto, J.F., and do Valle Marques, M. 2014. Analysis of the Caulobacter crescentus Zur regulon reveals novel insights in zinc acquisition by TonB-dependent outer membrane proteins. BMC Genomics 15, 734.
Meisenzahl, A.C., Shapiro, L., and Jenal, U. 1997. Isolation and characterization of a xylose-dependent promoter from Caulobacter crescentus. J. Bacteriol. 179, 592–600.
Neugebauer, H., Herrmann, C., Kammer, W., Schwarz, G., Nordheim, A., and Braun, V. 2005. ExbBD-dependent transport of maltodextrins through the novel MalA protein across the outer membrane of Caulobacter crescentus. J. Bacteriol. 187, 8300–8311.
Nierman, W.C., Feldblyum, T.V., Laub, M.T., Paulsen, I.T., Nelson, K.E., Eisen, J.A., Heidelberg, J.F., Alley, M.R., Ohta, N., Maddock, J.R., et al. 2001. Complete genome sequence of Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 98, 4136–4141.
Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656.
Noinaj, N., Guillier, M., Barnard, T.J., and Buchanan, S.K. 2010. TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. 64, 43–60.
Presley, G.N., Payea, M.J., Hurst, L.R., Egan, A.E., Martin, B.S., and Periyannan, G.R. 2014. Extracellular gluco-oligosaccharide degradation by Caulobacter crescentus. Microbiology 160, 635–645.
Ried, J.L. and Collmer, A. 1987. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in Gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57, 239–246.
Roberts, R.C., Mohr, C.D., and Shapiro, L. 1996. Developmental programs in bacteria. Curr. Top. Dev. Biol. 34, 207–257.
Ryan, K.R., Taylor, J.A., and Bowers, L.M. 2010. The BAM complex subunit BamE (SmpA) is required for membrane integrity, stalk growth and normal levels of outer membrane β-barrel proteins in Caulobacter crescentus. Microbiology 156, 742–756.
Sabri, S., Nielsen, L.K., and Vickers, C.E. 2013. Molecular control of sucrose utilization in Escherichia coli W, an efficient sucroseutilizing strain. Appl. Environ. Microbiol. 79, 478–487.
Saier, M.H. 2000. Families of transmembrane sugar transport proteins. Mol. Microbiol. 35, 699–710.
Salamov, V.S.A. and Solovyevand, A. 2011. Automatic annotation of microbial genomes and metagenomic sequences. pp. 61–78. In Li, R.W. (ed.), Metagenomics and its applications in agriculture. Nova Science Publishers, Hauppauge, NY,USA.
Schauer, K., Rodionov, D.A., and de Reuse, H. 2008. New substrates for TonB-dependent transport: do we only see the ‘tip of the iceberg’? Trends Biochem. Sci. 33, 330–338.
Shine, J. and Dalgarno, L. 1975. Determinant of cistron specificity in bacterial ribosomes. Nature 254, 34–38.
Thanbichler, M., Iniesta, A.A., and Shapiro, L. 2007. A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucleic Acids Res. 35, e137.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Modrak, S.K., Melin, M.E. & Bowers, L.M. SucA-dependent uptake of sucrose across the outer membrane of Caulobacter crescentus. J Microbiol. 56, 648–655 (2018). https://doi.org/10.1007/s12275-018-8225-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-018-8225-x