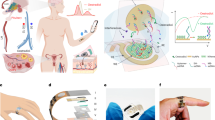
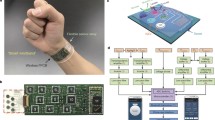
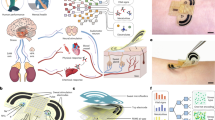
Abstract
The significant impact of stress on health necessitates accurate assessment methods, where traditional questionnaires lack reliability and objectivity. Current advancements like wearables with electrocardiogram (ECG) and galvanic skin response (GSR) sensors face accuracy and artifact challenges. Molecular biosensors detecting cortisol, a critical stress hormone, present a promising solution. However, existing cortisol assays, requiring saliva, urine, or blood, are complex, expensive, and unsuitable for continuous monitoring. Our study introduces a passive, molecularly imprinted polymer-radio-frequency (MIP-RF) wearable sensing system for real-time, non-invasive sweat cortisol assessment. This system is wireless, flexible, battery-free, reusable, environmentally stable, and designed for long-term monitoring, using an inductance-capacitance transducer. The transducer translates cortisol concentrations into resonant frequency shifts with high sensitivity (∼ 160 kHz/(log (µM))) across a physiological range of 0.025–1 µM. Integrated with near-field communication (NFC) for wireless and battery-free operation, and three-dimensional (3D)-printed microfluidic channel for in-situ sweat collection, it enables daily activity cortisol level tracking. Validation of cortisol circadian rhythm through morning and evening measurements demonstrates its effectiveness in tracking and monitoring sweat cortisol levels. A 28-day stability test and the use of cost-effective 3D nanomaterials printing enhance its economic viability and reusability. This innovation paves the way for a new era in realistic, on-demand health monitoring outside the laboratory, leveraging wearable technology for molecular stress biomarker detection.

Similar content being viewed by others
References
Singh, N. K.; Chung, S.; Sveiven, M.; Hall, D. A. Cortisol detection in undiluted human serum using a sensitive electrochemical structure-switching aptamer over an antifouling nanocomposite layer. ACS Omega 2021, 6, 27888–27897.
Wang, B.; Zhao, C. Z.; Wang, Z. Q.; Yang, K. A.; Cheng, X. B.; Liu, W. F.; Yu, W. Z.; Lin, S. Y.; Zhao, Y. C.; Cheung, K. M. et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 2022, 8, eabk0967.
Clow, A.; Hucklebridge, F.; Stalder, T.; Evans, P.; Thorn, L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010, 35, 97–103.
Nicolaides, N. C.; Charmandari, E.; Chrousos, G. P.; Kino, T. Circadian endocrine rhythms: The hypothalamic–pituitary–adrenal axis and its actions. Ann. N. Y. Acad. Sci. 2014, 1318, 71–80.
Steckl, A. J.; Ray, P. Stress biomarkers in biological fluids and their point-of-use detection. ACS Sens. 2018, 3, 2025–2044.
Venugopal, M.; Arya, S. K.; Chornokur, G.; Bhansali, S. A realtime and continuous assessment of cortisol in ISF using electrochemical impedance spectroscopy. Sens. Actuators A Phys. 2011, 172, 154–160.
Manenschijn, L.; Koper, J. W.; Lamberts, S. W. J.; Van Rossum, E. F. C. Evaluation of a method to measure long term cortisol levels. Steroids 2011, 76, 1032–1036.
Parlak, O.; Keene, S. T.; Marais, A.; Curto, V. F.; Salleo, A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 2018, 4, eaar2904.
Segerstrom, S. C.; Miller, G. E. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004, 130, 601–630.
Goh, J.; Pfeffer, J.; Zenios, S. A. The relationship between workplace stressors and mortality and health costs in the United States. Manage. Sci. 2016, 62, 608–628.
Laochai, T.; Yukird, J.; Promphet, N.; Qin, J. Q.; Chailapakul, O.; Rodthongkum, N. Non-invasive electrochemical immunosensor for sweat cortisol based on L-cys/AuNPs/ MXene modified thread electrode. Biosens. Bioelectron. 2022, 203, 114039.
Naik, A. R.; Zhou, Y. L.; Dey, A. A.; Arellano, D. L. G.; Okoroanyanwu, U.; Secor, E. B.; Hersam, M. C.; Morse, J.; Rothstein, J. P.; Carter, K. R. et al. Printed microfluidic sweat sensing platform for cortisol and glucose detection. Lab Chip 2022, 22, 156–169.
Monk, C. S.; Hart, K. A.; Berghaus, R. D.; Norton, N. A.; Moore, P. A.; Myrna, K. E. Detection of endogenous cortisol in equine tears and blood at rest and after simulated stress. Vet. Ophthalmol. 2014, 17, 53–60.
Sekar, M.; Sriramprabha, R.; Sekhar, P. K.; Bhansali, S.; Ponpandian, N.; Pandiaraj, M.; Viswanathan, C. Review-Towards wearable sensor platforms for the electrochemical detection of cortisol. J. Electrochem. Soc. 2020, 167, 067508.
Stevens, R. C.; Soelberg, S. D.; Near, S.; Furlong, C. E. Detection of cortisol in saliva with a flow-filtered, portable surface plasmon resonance biosensor system. Anal. Chem. 2008, 80, 6747–6751.
Ghaffari, R.; Yang, D. S.; Kim, J.; Mansour, A.; Wright, J. A.; Model, J. B.; Wright, D. E.; Rogers, J. A.; Ray, T. R. State of sweat: Emerging wearable systems for real-time, noninvasive sweat sensing and analytics. ACS Sens. 2021, 6, 2787–2801.
Huang, Z. Y.; Chen, H.; Ye, H. R.; Chen, Z. X.; Jaffrezic-Renault, N.; Guo, Z. Z. An ultrasensitive aptamer-antibody sandwich cortisol sensor for the noninvasive monitoring of stress state. Biosens. Bioelectron. 2021, 190, 113451.
Kinnamon, D.; Ghanta, R.; Lin, K. C.; Muthukumar, S.; Prasad, S. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep. 2017, 7, 13312.
An, J. E.; Kim, K. H.; Park, S. J.; Seo, S. E.; Kim, J.; Ha, S.; Bae, J.; Kwon, O. S. Wearable cortisol aptasensor for simple and rapid realtime monitoring. ACS Sens. 2022, 7, 99–108.
Shahar, T.; Tal, N.; Mandler, D. Molecularly imprinted polymer particles: Formation, characterization and application. Colloids Surf. A Physicochem. Eng. Aspects 2016, 495, 11–19.
Yeasmin, S.; Wu, B.; Liu, Y.; Ullah, A.; Cheng, L. J. Nano gold-doped molecularly imprinted electrochemical sensor for rapid and ultrasensitive Cortisol detection. Biosens. Bioelectron. 2022, 206, 114142.
Tang, W. X.; Yin, L.; Sempionatto, J. R.; Moon, J. M.; Teymourian, H.; Wang, J. Touch-based stressless cortisol sensing. Adv. Mater. 2021, 33, 2008465.
Li, Y. X.; Luo, L. X.; Kong, Y. Q.; Li, Y. J.; Wang, Q. S.; Wang, M. Q.; Li, Y.; Davenport, A.; Li, B. Recent advances in molecularly imprinted polymer-based electrochemical sensors. Biesens. Bioelectron. 2024, 249, 116018.
Betlem, K.; Down, M. P.; Foster, C. W.; Akthar, S.; Eersels, K.; Van Grinsven, B.; Cleij, T. J.; Banks, C. E.; Peeters, M. Development of a flexible MlP-based biosensor platform for the thermal detection of neurotransmitters. MRS Adv. 2018, 3, 1569–1574.
BelBruno, J. J. Molecularly imprinted polymers. Chem. Rev. 2019, 119, 94–119.
Ashley, J.; Shahbazi, M. A.; Kant, K.; Chidambara, V. A.; Wolff, A.; Bang, D. D.; Sun, Y. Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosens. Bioelectron. 2017, 91, 606–615.
Sheibani, S.; Capua, L.; Kamaei, S.; Akbari, S. S. A.; Zhang, J. R.; Guerin, H.; Ionescu, A. M. Extended gate field-effect-transistor for sensing cortisol stress hormone. Commun. Mater. 2021, 2, 10.
Lv, X. L.; Li, C. F.; Que, Y. H.; Li, G. F.; Hou, X. J.; Li, Y. J.; Li, L. F.; Sun, Y. B.; Guo, Y. S. Experimental demonstration of broadband impedance matching using coupled electromagnetic resonators. Sci. Rep. 2020, 10, 7437.
Piekarz, I.; Sorocki, J.; Górska, S.; Bartsch, H.; Rydosz, A.; Smolarz, R.; Wincza, K.; Gruszczynski, S. High sensitivity and selectivity microwave biosensor using biofunctionalized differential resonant array implemented in LTCC for Escherichia coli detection. Measurement 2023, 208, 112473.
Chen, R. G.; Van Wyk, J. D.; Wang, S.; Odendaal, W. G. Technologies and characteristics of integrated EMI filters for switch mode power supplies. In 2004 IEEE 35thAnnual Power Electronics Specialists Conference (IEEE Cat. No.04CH37551), Aachen, Germany, 2004, pp 4873–4880.
Khodapanahandeh, M.; Babaeihaselghobi, A.; Badri Ghavifekr, H. Design and simulation of a novel RF-MEMS tunable narrow band LC filter in the UHF band. Microsyst. Technol. 2021, 27, 325–334.
Niu, S. M.; Matsuhisa, N.; Beker, L.; Li, J. X.; Wang, S. H.; Wang, J. C.; Jiang, Y. W.; Yan, X. Z.; Yun, Y.; Burnett, W. et al. A wireless body area sensor network based on stretchable passive tags. Nat. Electron. 2019, 2, 361–368.
Takamatsu, T.; Sijie, Y.; Miyake, T. Wearable, implantable, parity-time symmetric bioresonators for extremely small biological signal monitoring. Adv. Mater. Technol. 2023, 8, 2201704.
Huang, Q. A.; Dong, L.; Wang, L. F. LC passive wireless sensors toward a wireless sensing platform: Status, prospects, and challenges. J. Microelectromech. Syst. 2016, 25, 822–841.
Nabavi, S.; Anabestani, H.; Bhadra, S. A printed paper-based RFID tag for wireless humidity sensing. In 2022 IEEE Sensors, Dallas, USA, 2022, pp, 1–4
Mazrouei, R.; Velasco, V.; Esfandyarpour, R. 3D-bioprinted all-inclusive bioanalytical platforms for cell studies. Sci. Rep. 2020, 10, 14669
Tavares-Negrete, J. A.; Babayigit, C.; Najafikoshnoo, S.; Lee, S. W.; Boyraz, O.; Esfandyarpour, R. A novel 3D-bioprinting technology of orderly extruded multi-materials via photopolymerization. Adv. Mater. Technol. 2023, 8, 2201926.
Velasco, V.; Joshi, K.; Chen, J. M.; Esfandyarpour, R. Personalized drug efficacy monitoring chip. Anal. Chem. 2019, 91, 14927–14935.
Esfandyarpour, R.; Esfandyarpour, H.; Javanmard, M.; Harris, J. S.; Davis, R. W. Electrical detection of protein biomarkers using nanoneedle biosensors. MRS OPL 2012, 1414, mrsf11–1414.
Esfandyarpour, R.; Javanmard, M.; Koochak, Z.; Esfandyarpour, H.; Harris, J. S.; Davis, R. W. Thin film nanoelectronic probe for protein detection. MRS OPL 2013, 1572, 1–6.
Yi, Q.; Najafikhoshnoo, S.; Das, P.; Noh, S.; Hoang, E.; Kim, T.; Esfandyarpour, R. All-3D-printed, flexible, and hybrid wearable bioelectronic tactile sensors using biocompatible nanocomposites for health monitoring. Adv. Mater. Technol. 2022, 7, 2101034.
Yi, Q.; Pei, X. C.; Das, P.; Qin, H. T.; Lee, S. W.; Esfandyarpour, R. A self-powered triboelectric MXene-based 3D-printed wearable physiological biosignal sensing system for on-demand, wireless, and real-time health monitoring. Nano Energy 2022, 101, 107511.
Nikbakhtnasrabadi, F.; Hosseini, E. S.; Dervin, S.; Shakthivel, D.; Dahiya, R. Smart bandage with inductor-capacitor resonant tank based printed wireless pressure sensor on electrospun poly-L-lactide nanofibers. Adv. Electron. Mater. 2022, 8, 2101348.
Ali, L.; Wang, C.; Meng, F. Y.; Adhikari, K. K.; Wei, Y. C.; Zhao, M. High-sensitivity accurate characterization of complex permittivity using inter-digital capacitor-based planar microwave sensor. In 2027 4th International Conference on Information Communication and Signal Processing (ICICSP), Shanghai, China, 2021, pp, 295–298
Sontimuang, C.; Suedee, R.; Dickert, F. Interdigitated capacitive biosensor based on molecularly imprinted polymer for rapid detection of Hev b1 latex allergen. Anal. Biochem. 2011, 410, 224–233.
Das, P.; Najafikhoshnoo, S.; Tavares-Negrete, J. A.; Yi, Q.; Esfandyarpour, R. An in-vivo-mimicking 3D lung cancer-on-a-chip model to study the effect of external stimulus on the progress and inhibition of cancer metastasis. Bioprinting 2022, 28, e00243.
Kim, H.; Kim, Y. S.; Mahmood, M.; Kwon, S.; Zavanelli, N.; Kim, H. S.; Rim, Y. S.; Epps, F.; Yeo, W. H. Fully integrated, stretchable, wireless skin-conformal bioelectronics for continuous stress monitoring in daily life. Adv. Sci. 2020, 7, 2000810.
Kalra, A.; Lowe, A.; Al-Jumaily, A. Mechanical behaviour of skin: A review. J. Mater. Sci. Eng. 2016, 5, 1000254.
Wang, B. H.; Huang, W.; Chi, L. F.; Al-Hashimi, M.; Marks, T. J.; Facchetti, A. High-k gate dielectrics for emerging flexible and stretchable electronics. Chem. Rev. 2018, 118, 5690–5754.
Xu, Y. D.; Sun, B. H.; Ling, Y.; Fei, Q. H.; Chen, Z. Y.; Li, X. P.; Guo, P. J.; Jeon, N.; Goswami, S.; Liao, Y. X. et al. Multiscale porous elastomer substrates for multifunctional on-skin electronics with passive-cooling capabilities. Proc. Natl. Acad. Sci. USA 2020, 117, 205–213.
Sun, D.; Zhang, Y.; Liu, Y. F.; Wang, Z. G.; Chen, X. C.; Meng, Z. Y.; Kang, S. F.; Zheng, Y. Y.; Cui, L. F.; Chen, M. L. et al. In-situ homodispersely immobilization of Ag@AgCl on chloridized g-C3N4 nanosheets as an ultrastable plasmonic photocatalyst. Chem. Eng. J. 2020, 384, 123259.
NajafiKhoshnoo, S.; Kim, T.; Tavares-Negrete, J. A.; Pei, X. C.; Das, P.; Lee, S. W.; Rajendran, J.; Esfandyarpour, R. A 3D nanomaterials-printed wearable, battery-free, biocompatible, flexible, and wireless pH sensor system for real-time health monitoring. Adv. Mater. Technol. 2023, 8, 2201655.
Lee, S. W.; Pei, X. C.; Rajendran, J.; Esfandyarpour, R. A wireless and battery-free wearable pressure sensing system for human-machine interaction and health monitoring. IEEE J. Flexible Electron. 2023, 2, 439–447.
Mei, H.; Zhao, X.; Gui, X. C.; Lu, D. W.; Han, D. Y.; **ao, S. S.; Cheng, L. F. SiC encapsulated Fe@CNT ultra-high absorptive shielding material for high temperature resistant EMI shielding. Ceram. Int. 2019, 45, 17144–17151.
Zhu, X. H.; Liu, K.; Lu, Z. B.; Xu, Y. P.; Qi, S. S.; Zhang, G. G. Effect of oxygen atoms on graphene: Adsorption and do**. Phys. E Low Dimens. Syst. Nanostruct. 2020, 117, 113827.
Rajendran, J. Amperometric determination of salivary thiocyanate using electrochemically fabricated poly (3, 4-ethylenedioxythiophene)/MXene hybrid film. J. Hazard. Mater. 2023, 449, 130979.
Al-Hetlani, E.; D’Cruz, B.; Amin, M. O. A 3D miniaturized solid-state chemiluminescence sensor based on ruthenium functionalized polymeric monolith for the detection of pharmaceutical drugs. J. Mater. Sci. 2020, 55, 13232–13243.
Gillan, L.; Jansson, E. Molecularly imprinted polymer on roll-to-roll printed electrodes as a single use sensor for monitoring of cortisol in sweat. Flex. Print. Electron. 2022, 7, 025014.
Yulianti, E. S.; Rahman, S. F.; Whulanza, Y. Molecularly imprinted polymer-based sensor for electrochemical detection of cortisol. Biosensors 2022, 12, 1090.
Semitekolos, D.; Kainourgios, P.; Jones, C.; Rana, A.; Koumoulos, E. P.; Charitidis, C. A. Advanced carbon fibre composites via poly methacrylic acid surface treatment; surface analysis and mechanical properties investigation. Compos. Part B Eng. 2018, 155, 237–243.
**ng, Y.; Sun, X. M.; Li, B. H. Poly(methacrylic acid)-modified chitosan for enhancement adsorption of water-soluble cationic dyes. Polym. Eng. Sci. 2009, 49, 272–280.
Liu, D.; Pan, J. L.; Tang, J. H.; Lian, N. Preparation of polymethacrylate monolith modified with cysteine for the determination of Cr(III) ions. RSC Adv. 2018, 8, 24906–24912.
Chawla, V.; Ha, D. S. An overview of passive RFID. IEEE Commun. Mag. 2007, 45, 11–17.
Elbasheir, M. S.; Saeed, R. A.; Edam, S. Measurement and simulation-based exposure assessment at a far-field for a multitechnology cellular site up to 5G NR. IEEE Access 2022, 10, 56888–56900.
Fernández, M.; Guerra, D.; Gil, U.; Trigo, I.; Peña, I.; Arrinda, A. Measurements and analysis of temporal and spatial variability of WiFi exposure levels in the 2.4 GHz frequency band. Measurement 2020, 149, 106970.
Wagih, M.; Komolafe, A.; Weddell, A. S.; Beeby, S. Broadband compact substrate-independent textile wearable antenna for simultaneous near- and far-field wireless power transmission. IEEE Open J. Antennas Propag. 2022, 3, 398–411.
Yang, C. P.; Su, Q. P.; Zheng, S. B.; Nori, F. Crosstalk-insensitive method for simultaneously coupling multiple pairs of resonators. Phys. Rev. A 2016, 93, 042307.
Lathiya, P.; Wang, J. Near-field communications (NFC) for wireless power transfer (WPT): An overview. In Wireless Power Transfer-Recent Development, Applications and New Perspectives. Zellagui, M., Ed.; IntechOpen: London, 2021, pp, 95–122.
Ofosu Addo, E.; Kommey, B.; Selasi Agbemenu, A.; Kumbong, H. On the design and implementation of efficient antennas for high frequency-radio frequency identification read/write devices. Eng. Rep. 2021, 3, e12407.
Unger, C.; Lieberzeit, P. A. Molecularly imprinted thin film surfaces in sensing: Chances and challenges. React. Funct. Polym. 2021, 161, 104855.
Ansell, R. J. Characterization of the binding properties of molecularly imprinted polymers. In Molecularly Imprinted Polymers in Biotechnology. Mattiasson, B.; Ye, L., Eds.; Springer: Cham, 2015, pp 51–93
Hourlier-Fargette, A.; Schon, S.; Xue, Y. G.; Avila, R.; Li, W. H.; Gao, Y. W.; Liu, C.; Kim, S. B.; Raj, M. S.; Fields, K. B. et al. Skin-interfaced soft microfluidic systems with modular and reusable electronics for in situ capacitive sensing of sweat loss, rate and conductivity. Lab Chip 2020, 20, 4391–4403.
Mustafa, M.; Rizwan, M.; Kashif, M.; Khan, T.; Waseem, M.; Annuk, A. LC passive wireless sensor system based on two switches for detection of triple parameters. Sensors 2022, 22, 3024.
Raul, J. S.; Cirimele, V.; Ludes, B.; Kintz, P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin. Biochem. 2004, 37, 1105–1111.
Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. H. M. The detection of cortisol in human sweat: Implications for measurement of cortisol in hair. Ther. Drug Monit. 2014, 36, 30–34.
Lu, N. S.; Lu, C.; Yang, S. X.; Rogers, J. Highly sensitive skin-mountable strain gauges based entirely on elastomers. Adv. Funct. Mater. 2012, 22, 4044–4050.
Hubert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hddogenn sensors—A review. Sens. Actuators B: Chem. 2011, 157, 329–352.
Kim, T.; Yi, Q.; Hoang, E.; Esfandyarpour, R. A 3D printed wearable bioelectronic patch for multi-sensing and in situ sweat electrolyte monitoring. Adv. Mater. Technol. 2021, 6, 2001021.
Joshi, K.; Javani, A.; Park, J.; Velasco, V.; Xu, B. Z.; Razorenova, O.; Esfandyarpour, R. A machine learning-assisted nanoparticle-printed biochip for real-time single cancer cell analysis. Adv. Biosyst. 2020, 4, 2000160.
Acknowledgements
This work was supported by the start-up funds provided to R. E. by the Henry Samueli School of Engineering and the Department of Electrical Engineering and Computer Science at the University of California, Irvine.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Supplementary material, approximately 34.7 MB.
12274_2024_6738_MOESM2_ESM.pdf
Electronic Supplementary Material: A passive, reusable, and resonating wearable sensing system for on-demand, non-invasive, and wireless molecular stress biomarker detection
Rights and permissions
About this article
Cite this article
Chakoma, S., Pei, X., Qin, H. et al. A passive, reusable, and resonating wearable sensing system for on-demand, non-invasive, and wireless molecular stress biomarker detection. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6738-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12274-024-6738-7