Abstract
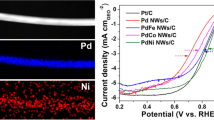
Increasing the utilization efficiency of platinum is critical for advancing proton exchange-membrane fuel cells (PEMFCs). Despite extensive research on catalysts for the cathodic oxygen reduction reaction (ORR), develo** highly active and durable Pt-based catalysts that can suppress surface dealloying in corrosive acid conditions remains challenging. Herein, we report a facile synthesis of bimetallic ultrathin PtM (M = Mo, W, and Cr) nanowires (NWs) composed of group VI B transition metal atomic sites anchored on the surface. These NWs possess uniform sizes and well-controlled atomic arrangements. Compared to PtW and PtCr catalysts, the PtMo0.05 NWs exhibit the highest half-wave potential of 0.935 V and a mass activity of 1.43 A·mgPt−1. Remarkably, they demonstrate a remarkable 23.8-fold enhancement in mass activity compared to commercial Pt/C for ORR, surpassing previously reported Pt-based catalysts. Additionally, the PtMo NWs cathode in membrane electrode assembly tests achieves a remarkable peak power density of 1.443 W·cm−2 (H2-O2 conditions at 80 °C), which is 1.09 times that of commercial Pt/C. The ligand effect in the bimetallic surface not only facilitates strong coupling between Mo (4d) and Pt (5d) atomic orbitals to hinder atom leaching but also modulates the d-states of active site, significantly optimizing the adsorption of key oxygen (⋆O and ⋆OH) species and accelerating the rate-determining step in ORR pathways.

Similar content being viewed by others
References
Han, A. L.; Sun, W. M.; Wan, X.; Cai, D. D.; Wang, X. J.; Li, F.; Shui, J. L.; Wang, D. S. Construction of Co4 atomic clusters to enable Fe-N4 motifs with highly active and durable oxygen reduction performance. Angew. Chem., Int. Ed. 2023, 62, e202303185.
Li, J. J.; **a, W.; Tang, J.; Gao, Y.; Jiang, C.; Jia, Y. N.; Chen, T.; Hou, Z. F.; Qi, R. J.; Jiang, D. et al. Metal-organic framework-derived graphene mesh: A robust scaffold for highly exposed Fe-N4 active sites toward an excellent oxygen reduction catalyst in acid media. J. Am. Chem. Soc. 2022, 144, 9280–9291.
**ao, F.; Wang, Q.; Xu, G. L.; Qin, X. P.; Hwang, I.; Sun, C. J.; Liu, M.; Hua, W.; Wu, H. W.; Zhu, S. Q. et al. Atomically dispersed Pt and Fe sites and Pt-Fe nanoparticles for durable proton exchange membrane fuel cells. Nat. Catal. 2022, 5, 503–512.
Sun, Y. Y.; Polani, S.; Luo, F.; Ott, S.; Strasser, P.; Dionigi, F. Advancements in cathode catalyst and cathode layer design for proton exchange membrane fuel cells. Nat. Commun. 2021, 12, 5984.
Jiao, K.; Xuan, J.; Du, Q.; Bao, Z. M.; **e, B.; Wang, B. W.; Zhao, Y.; Fan, L. H.; Wang, H. Z.; Hou, Z. J. et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369.
Fan, J. T.; Chen, M.; Zhao, Z. L.; Zhang, Z.; Ye, S. Y.; Xu, S. Y.; Wang, H. J.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486.
Chen, Y. P.; Zheng, X. S.; Cai, J. Y.; Zhao, G. Q.; Zhang, B. X.; Luo, Z. X.; Wang, G. M.; Pan, H. G.; Sun, W. P. Sulfur do** triggering enhanced Pt-N coordination in graphitic carbon nitride-supported Pt electrocatalysts toward efficient oxygen reduction reaction. ACS Catal. 2022, 12, 7406–7414.
Lu, B. A.; Shen, L. F.; Liu, J.; Zhang, Q. H.; Wan, L. Y.; Morris, D. J.; Wang, R. X.; Zhou, Z. Y.; Li, G.; Sheng, T. et al. Structurally disordered phosphorus-doped Pt as a highly active electrocatalyst for an oxygen reduction reaction. ACS Catal. 2021, 11, 355–363.
Han, A. L.; Wang, X. J.; Tang, K.; Zhang, Z. D.; Ye, C. L.; Kong, K. J.; Hu, H. B.; Zheng, L. R.; Jiang, P.; Zhao, C. X. et al. An adjacent atomic platinum site enables single-atom iron with high oxygen reduction reaction performance. Angew. Chem., Int. Ed. 2021, 60, 19262–19271.
Li, X.; He, Y. H.; Cheng, S. B.; Li, B. Y.; Zeng, Y. C.; **e, Z. H.; Meng, Q. P.; Ma, L.; Kisslinger, K.; Tong, X. et al. Atomic structure evolution of Pt-Co binary catalysts: Single metal sites versus intermetallic nanocrystals. Adv. Mater. 2021, 33, 2106371.
Gao, L.; Li, X. X.; Yao, Z. Y.; Bai, H. J.; Lu, Y. F.; Ma, C.; Lu, S. F.; Peng, Z. M.; Yang, J. L.; Pan, A. L. et al. Unconventional p-d hybridization interaction in PtGa ultrathin nanowires boosts oxygen reduction electrocatalysis. J. Am. Chem. Soc. 2019, 141, 18083–18090.
Zaman, S.; Huang, L.; Douka, A. I.; Yang, H.; You, B.; **a, B. Y. Oxygen reduction electrocatalysts toward practical fuel cells: Progress and perspectives. Angew. Chem. 2021, 133, 17976–17996.
Zhuang, Z. C.; Li, Y.; Li, Y. H.; Huang, J. Z.; Wei, B.; Sun, R.; Ren, Y. J.; Ding, J.; Zhu, J. X.; Lang, Z. Q. et al. Atomically dispersed nonmagnetic electron traps improve oxygen reduction activity of perovskite oxides. Energy Environ. Sci., 2021, 14, 1016–1028.
Zhuang, Z. C.; **a, L. X.; Huang, J. Z.; Zhu, P.; Li, Y.; Ye, C. L.; **a, M. G.; Yu, R. H.; Lang, Z. Q.; Zhu, J. X. et al. Continuous modulation of electrocatalytic oxygen reduction activities of single-atom catalysts through p-n junction rectification. Angew. Chem., Int. Ed. 2023, 62, e202212335.
Cheng, Q. Q.; Yang, S.; Fu, C. H.; Zou, L. L.; Zou, Z. Q.; Jiang, Z.; Zhang, J. L.; Yang, H. High-loaded sub-6 nm Pt1Co1 intermetallic compounds with highly efficient performance expression in PEMFCs. Energy Environ. Sci. 2022, 15, 278–286.
Chang, F. F.; Bai, Z. Y.; Li, M.; Ren, M. Y.; Liu, T. C.; Yang, L.; Zhong, C. J.; Lu, J. Strain-modulated platinum-palladium nanowires for oxygen reduction reaction. Nano Lett. 2020, 20, 2416–2422.
Hu, Y. Z.; Guo, X. Y.; Shen, T.; Zhu, Y.; Wang, D. L. Hollow porous carbon-confined atomically ordered PtCo3 intermetallics for an efficient oxygen reduction reaction. ACS Catal. 2022, 12, 5380–5387.
Zaman, S.; Su, Y. Q.; Dong, C. L.; Qi, R. J.; Huang, L.; Qin, Y. Y.; Huang, Y. C.; Li, F. M.; You, B.; Guo, W. et al. Scalable molten salt synthesis of platinum alloys planted in metal-nitrogen-graphene for efficient oxygen reduction. Angew. Chem., Int. Ed. 2022, 61, e202115835.
He, T. O.; Wang, W. C.; Yang, X. L.; Shi, F. L.; Ye, Z. Y.; Zheng, Y. Z.; Li, F.; Wu, J. B.; Yin, Y. D.; **, M. S. Deposition of atomically thin Pt shells on amorphous palladium phosphide cores for enhancing the electrocatalytic durability. ACS Nano 2021, 15, 7348–7356.
**, H.; Xu, Z. W.; Hu, Z. Y.; Yin, Z. W.; Wang, Z.; Deng, Z.; Wei, P.; Feng, S. H.; Dong, S. H.; Liu, J. F. et al. Mesoporous Pt@Pt-skin Pt3Ni core–shell framework nanowire electrocatalyst for efficient oxygen reduction. Nat. Commun. 2023, 14, 1518.
Liu, Z. Y.; Zhao, Z. P.; Peng, B. S.; Duan, X. F.; Huang, Y. Beyond extended surfaces: Understanding the oxygen reduction reaction on nanocatalysts. J. Am. Chem. Soc. 2020, 142, 17812–17827.
Wang, L. G.; Wu, J. B.; Wang, S. W.; Liu, H.; Wang, Y.; Wang D. S. The reformation of catalyst: From a trial-and-error synthesis to rational design. Nano Res. 2024, 17, 3261–3301
Feng, Q. C.; Wang, X. L.; Klingenhof, M.; Heggen, M.; Strasser, P. Low-Pt NiNC-supported PtNi nanoalloy oxygen reduction reaction electrocatalysts—In situ tracking of the atomic alloying process. Angew. Chem., Int. Ed. 2022, 61, e202203728.
Wei, M.; Huang, L.; Li, L. B.; Ai, F.; Su, J. Z.; Wang, J. K. Coordinatively unsaturated PtCo flowers assembled with ultrathin nanosheets for enhanced oxygen reduction. ACS Catal. 2022, 12, 6478–6485.
Tu, W. Z.; Luo, W. J.; Chen, C. L.; Chen, K.; Zhu, E. B.; Zhao, Z. P.; Wang, Z. L.; Hu, T.; Zai, H. C.; Ke, X. X. et al. Tungsten as “adhesive” in Pt2CuW0.25 ternary alloy for highly durable oxygen reduction electrocatalysis. Adv. Funct. Mater. 2020, 30, 1908230.
Zhang, Z.; Yang, S.; Jiang, R.; Sheng, T.; Shi, C.; Chen, Y.; Wang, L. Intensifying uneven charge distribution via geometric distortion engineering in atomically dispersed M-Nx/S sites for efficient oxygen electroreduction. Nano Res. 2022, 15, 8928–8935.
Zhu, S. Q.; Qin, X. P.; Yao, Y.; Shao, M. H. pH-dependent hydrogen and water binding energies on platinum surfaces as directly probed through surface-enhanced infrared absorption spectroscopy. J. Am. Chem. Soc. 2020, 142, 8748–8754.
Wandlowski, T.; Ataka, K.; Pronkin, S.; Diesing, D. Surface enhanced infrared spectroscopy-Au (111-20 nm)/sulphuric acid-new aspects and challenges. Electrochim. Acta 2004, 49, 1233–1247.
Li, P.; Jiao, Y. Z.; Ruan, Y. E.; Fei, H. G.; Men, Y. N.; Guo, C. L.; Wu, Y. E.; Chen, S. L. Revealing the role of double-layer microenvironments in pH-dependent oxygen reduction activity over metal-nitrogen-carbon catalysts. Nat. Commun. 2023, 14, 6936.
**ao, Z. H.; Chen, Y. G.; Wu, R. J.; He, Y. W.; Shi, C. F.; Wang, L. Y. OH regulator of highly dispersed Ru sites on host Pd nanocrystals for selective ethanol electro-oxidation. Nano Res. 2024, 17, 3863–3871.
Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J. K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 2018, 118, 2302–2312.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 22275009), SINOPEC (contact No. 421028), and Fundamental Research Funds for the Central Universities (No. XK2020-02). We thank the BL14W1 station in Shanghai Synchrotron Radiation Facility (SSRF). The authors would like to express their gratitude to Prof. Zhongbin Zhuang, Prof. Wei Zhu, and Mr. Cheng** Chen from BUCT for their assistance with the MEA test.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2024_6528_MOESM1_ESM.pdf
Ligand effect in surface atomic sites of group VI B transition metals on ultrathin Pt nanowires for enhanced oxygen reduction
Rights and permissions
About this article
Cite this article
He, Y., Chen, Y., Wu, R. et al. Ligand effect in surface atomic sites of group VI B transition metals on ultrathin Pt nanowires for enhanced oxygen reduction. Nano Res. 17, 5298–5304 (2024). https://doi.org/10.1007/s12274-024-6528-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-024-6528-2