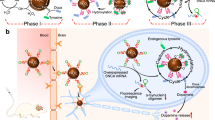
Abstract
In recent years, neurodegenerative diseases, such as Parkinson’s or Alzheimer’s diseases, are rapidly rising in prevalence. The main hallmark of Parkinson’s disease is the falling levels of neurotransmitter dopamine in the mid-brain with dopaminergic neurons losing. Typical therapeutic solutions, including drugs, deep brain stimulation, and cell transplantation, can only alleviate the symptoms of Parkinson’s disease. It is a tremendous challenge to reverse the function degeneration of the crucial dopaminergic neurons. Herein, we develop a core-satellite-like nanoassembly (PDA-AFn (by integrating polydopamine nanoparticles and apoferritin)) to raise the expression of tyrosine hydroxylase (TH), a rate-limiting enzyme in the formation of the dopamine. Both components in the nanoassembly could cooperate with each other, not only elaborately regulate the iron homeostasis and redox microenvironment, but also utilize excessive reactive oxygen species (ROS) and iron ions in the damaged neurons to supply extra dopamine and enhance TH activity, and consequently restore the function of the degenerated neurons. Remarkably, the nanoassembly-treatment relieves the dyskinesia and dramatical increases the tyrosine hydroxylase and dopamine level in the midbrain of Parkinson’s disease model mice. It is an explicit yet inspiring advance in treatment of the neurodegeneration.

Similar content being viewed by others
References
Lotharius, J.; Brundin, P. Pathogenesis of Parkinson’s disease: Dopamine, vesicles and α-synuclein. Nat. Rev. Neurosci. 2002, 3, 932–942.
da Silva, J. A.; Tecuapetla, F.; Paixão, V.; Costa, R. M. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 2018, 554, 244–248.
Nobili, A.; Latagliata, E. C.; Viscomi, M. T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F. R.; Marino, R.; Federici, M. et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 2017, 8, 14727.
Kalia, L. V.; Lang, A. E. Parkinson’s disease. Lancet 2015, 386, 896–912.
Chaudhuri, K. R.; Schapira, A. H. V. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474.
Kim, T.; Kim, H. J.; Choi, W.; Lee, Y. M.; Pyo, J. H.; Lee, J.; Kim, J.; Kim, J.; Kim, J. H.; Kim, C. et al. Deep brain stimulation by blood-brain-barrier-crossing piezoelectric nanoparticles generating current and nitric oxide under focused ultrasound. Nat. Biomed. Eng. 2023, 7, 149–163.
Howe, M. W.; Dombeck, D. A. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 2016, 535, 505–510.
Lee, T.; Cai, L. X.; Lelyveld, V. S.; Hai, A.; Jasanoff, A. Molecular-level functional magnetic resonance imaging of dopaminergic signaling. Science 2014, 344, 533–535.
Tiklová, K.; Björklund, Å. K.; Lahti, L.; Fiorenzano, A.; Nolbrant, S.; Gillberg, L.; Volakakis, N.; Yokota, C.; Hilscher, M. M.; Hauling, T. et al. Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat. Commun. 2019, 10, 581.
Feng, P. J.; Chen, Y. L.; Zhang, L.; Qian, C. G.; **ao, X. Z.; Han, X.; Shen, Q. D. Near-infrared fluorescent nanoprobes for revealing the role of dopamine in drug addiction. ACS Appl. Mater. Interfaces 2018, 10, 4359–4368.
Raza, C.; Anjum, R.; Shakeel, N. U. A. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019, 226, 77–90.
Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C. W.; Merchant, K. M.; Bezard, E. et al. Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2015, 14, 855–866.
de Bie, R. M. A.; Clarke, C. E.; Espay, A. J.; Fox, S. H.; Lang, A. E. Initiation of pharmacological therapy in Parkinson’s disease: When, why, and how. Lancet Neurol. 2020, 19, 452–461.
Ogawa, N. Levodopa and dopamine agonists in the treatment of Parkinson’s disease: Advantages and disadvantages. Eur. Neurol. 1994, 34, 20–28.
Kriks, S.; Shim, J. W.; Piao, J.; Ganat, Y. M.; Wakeman, D. R.; **e, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551.
Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H. et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017, 548, 592–596.
Zhang, Z. R.; Wang, F. Y.; Song, M. J. The cell repair research of spinal cord injury: A review of cell transplantation to treat spinal cord injury. J. Neurorestoratol. 2019, 7, 55–62.
Rao, F. W.; Zhang, L.; Wessel, J.; Zhang, K. X.; Wen, G.; Kennedy, B. P.; Rana, B. K.; Das, M.; Rodriguez-Flores, J. L.; Smith, D. W. et al. Tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis: Discovery of common human genetic variants governing transcription, autonomic activity, and blood pressure in vivo. Circulation 2007, 116, 993–1006.
Morales, M.; Margolis, E. B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85.
Lammel, S.; Lim, B. K.; Malenka, R. C. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 2014, 76, 351–359.
Jiao, S. S.; Gurevich, V.; Wolff, J. A. Long-term correction of rat model of Parkinson’s disease by gene therapy. Nature 1993, 362, 450–453.
Du, X. Y.; Iacovitti, L. Multiple signaling pathways direct the initiation of tyrosine hydroxylase gene expression in cultured brain neurons. Mol. Brain Res. 1997, 50, 1–8.
Du, X. Y.; Stull, N. D.; Iacovitti, L. Brain-derived neurotrophic factor works coordinately with partner molecules to initiate tyrosine hydroxylase expression in striatal neurons. Brain Res. 1995, 680, 229–233.
Tavakol, S.; Musavi, S. M. M.; Tavakol, B.; Hoveizi, E.; Ai, J.; Rezayat, S. M. Noggin along with a self-assembling peptide nanofiber containing long motif of laminin induces tyrosine hydroxylase gene expression. Mol. Neurobiol. 2017, 54, 4609–4616.
Zhao, D.; Feng, P. J.; Liu, J. H.; Dong, M.; Shen, X. Q.; Chen, Y. X.; Shen, Q. D. Electromagnetized -nanoparticle-modulated neural plasticity and recovery of degenerative dopaminergic neurons in the mid-brain. Adv. Mater. 2020, 32, 2003800.
El Mestikawy, S.; Glowinski, J.; Hamon, M. Tyrosine hydroxylase activation in depolarized dopaminergic terminals-involvement of Ca2+-dependent phosphorylation. Nature 1983, 302, 830–832.
Haycock, J. W.; Ahn, N. G.; Cobb, M. H.; Krebs, E. G. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc. Natl. Acad. Sci. USA 1992, 89, 2365–2369.
Burbulla, L. F.; Song, P. P.; Mazzulli, J. R.; Zampese, E.; Wong, Y. C.; Jeon, S.; Santos, D. P.; Blanz, J.; Obermaier, C. D.; Strojny, C. et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 2017, 357, 1255–1261.
McCormack, A. L.; Atienza, J. G.; Johnston, L. C.; Andersen, J. K.; Vu, S.; Di Monte, D. A. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J. Neurochem. 2005, 93, 1030–1037.
Kim, G. H.; Kim, J. E.; Rhie, S. J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340.
Dela Cruz, C. P.; Revilla, E.; Venero, J. L.; Ayala, A.; Cano, J.; MacHado, A. Oxidative inactivation of tyrosine hydroxylase in substantia nigra of aged rat. Free Radical Biol. Med. 1996, 20, 53–61.
Rostrup, M.; Fossbakk, A.; Hauge, A.; Kleppe, R.; Gnaiger, E.; Haavik, J. Oxygen dependence of tyrosine hydroxylase. Amino Acids 2008, 34, 455–464.
Rodríguez-Gómez, J. A.; Venero, J. L.; Vizuete, M. L.; Cano, J.; Machado, A. Deprenyl induces the tyrosine hydroxylase enzyme in the rat dopaminergic nigrostriatal system. Mol. Brain Res. 1997, 46, 31–38.
Knörle, R. Neuromelanin in Parkinson’s Disease: From Fenton reaction to calcium signaling. Neurotox. Res. 2018, 33, 515–522.
Hirsch, E. C.; Brandel, J. P.; Galle, P.; Javoyagid, F.; Agid, Y. Iron and aluminum increase in the Substantia Nigra of patients with Parkinson’s disease: An X-ray-Microanalysis. J. Neurochem. 1991, 56, 446–451.
Frantom, P. A.; Seravalli, J.; Ragsdale, S. W.; Fitzpatrick, P. F. Reduction and oxidation of the active site iron in tyrosine hydroxylase: Kinetics and specificity. Biochemistry 2006, 45, 2372–2379.
Zhou, J.; Zhang, Z. Y.; Joseph, J.; Zhang, X. C.; Ferdows, B. E.; Patel, D. N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D. et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 1, 20210011.
Zhang, Y. Y.; Tang, Z. W.; Wang, J.; Wu, H.; Lin, C. T.; Lin, Y. H. Apoferritin nanoparticle: A novel and biocompatible carrier for enzyme immobilization with enhanced activity and stability. J. Mater. Chem. 2011, 21, 17468–17475.
Jutz, G.; van Rijn, P.; Miranda, B. S.; Böker, A. Ferritin: A versatile building block for bionanotechnology. Chem. Rev. 2015, 115, 1653–1701.
Zhang, N.; Yu, X. Q.; **e, J. X.; Xu, H. M. New insights into the role of ferritin in iron homeostasis and neurodegenerative diseases. Mol. Neurobiol. 2021, 58, 2812–2823.
Balla, G.; Jacob, H. S.; Balla, J.; Rosenberg, M.; Nath, K.; Apple, F.; Eaton, J. W.; Vercellotti, G. M. Ferritin: A cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992, 267, 18148–18153.
d’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C. T.; Buehler, M. J. Polydopamine and eumelanin: From structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550.
Kang, K.; Choi, I. S.; Nam, Y. A biofunctionalization scheme for neural interfaces using polydopamine polymer. Biomaterials 2011, 32, 6374–6380.
Li, Y. W.; **e, Y. J.; Wang, Z.; Zang, N. Z.; Carniato, F.; Huang, Y. R.; Andolina, C. M.; Parent, L. R.; Ditri, T. B.; Walter, E. D. et al. Structure and function of iron-loaded synthetic melanin. ACS Nano 2016, 10, 10186–10194.
Zhang, Z. Y.; Zhou, J.; Liu, C.; Zhang, J. M.; Shibata, Y.; Kong, N.; Corbo, C.; Harris, M. B.; Tao, W. Emerging biomimetic nanotechnology in orthopedic diseases: Progress, challenges, and opportunities. Trends Chem. 2022, 4, 420–436.
Battaglini, M.; Marino, A.; Carmignani, A.; Tapeinos, C.; Cauda, V.; Ancona, A.; Garino, N.; Vighetto, V.; La Rosa, G.; Sinibaldi, E. et al. Polydopamine nanoparticles as an organic and biodegradable multitasking tool for neuroprotection and remote neuronal stimulation. ACS Appl. Mater. Interfaces. 2020, 12, 35782–35798.
Bao, X. F.; Zhao, J. H.; Sun, J.; Hu, M.; Yang, X. R. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano 2018, 12, 8882–8892.
Chasteen, N. D.; Harrison, P. M. Mineralization in ferritin: An efficient means of iron storage. J. Struct. Biol. 1999, 126, 182–194.
Harrison, P. M. The structure of apoferritin: Molecular size, shape and symmetry from X-ray data. J. Mol. Biol. 1963, 6, 404–422.
Aguilera-Garrido, A.; Del Castillo-Santaella, T.; Yang, Y.; Galisteo-González, F.; Gálvez-Ruiz, M. J.; Molina-Bolívar, J. A.; Holgado-Terriza, J. A.; Cabrerizo-Vílchez, M. Á.; Maldonado-Valderrama, J. Applications of serum albumins in delivery systems: Differences in interfacial behaviour and interacting abilities with polysaccharides. Adv. Colloid Interface Sci. 2021, 290, 102365.
Wang, Y.; Tong, Q.; Ma, S. R.; Zhao, Z. X.; Pan, L. B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y. et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct Target Ther. 2021, 6, 77.
Reinert, A.; Morawski, M.; Seeger, J.; Arendt, T.; Reinert, T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 2019, 20, 25.
Wu, W. C.; Hu, D. N.; Gao, H. X.; Chen, M.; Wang, D. W.; Rosen, R.; McCormick, S. A. Subtoxic levels hydrogen peroxide-induced production of interleukin-6 by retinal pigment epithelial cells. Mol. Vis. 2010, 16, 1864–1873.
Honarmand Ebrahimi, K.; Bill, E.; Hagedoorn, P. L.; Hagen, W. R. The catalytic center of ferritin regulates iron storage via Fe(II)–Fe(III) displacement. Nat. Chem. Biol. 2012, 8, 941–948.
Denton, T.; Howard, B. D. A dopaminergic cell line variant resistant to the neurotoxin 1-Methyl-4-Phenyl-1, 2, 3, 6-tetrahydropyridine. J. Neurochem. 1987, 49, 622–630.
Ochu, E. E.; Rothwell, N. J.; Waters, C. M. Caspases mediate 6-hydroxydopamine-induced apoptosis but not necrosis in PC12 Cells. J. Neurochem. 1998, 70, 2637–2640.
Evetts, K. D.; Uretsky, N. J.; Iversen, L. L.; Iversen, S. D. Effects of 6-hydroxydopamine on CNS catecholamines, spontaneous motor activity and amphetamine induced hyperactivity in rats. Nature 1970, 225, 961–962.
Heikkila, R. E.; Cohen, G. 6-Hydroxydopamine: Evidence for superoxide radical as an oxidative intermediate. Science 1973, 181, 456–457.
Cheng, W.; Zeng, X. W.; Chen, H. Z.; Li, Z. M.; Zeng, W. F.; Mei, L.; Zhao, Y. L. Versatile polydopamine platforms: Synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano 2019, 13, 8537–8565.
Di Salvio, M.; Di Giovannantonio, L. G.; Acampora, D.; Prosperi, R.; Omodei, D.; Prakash, N.; Wurst, W.; Simeone, A. Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat. Neurosci. 2010, 13, 1481–1488.
You, L. H.; Wang, J.; Liu, T. Q.; Zhang, Y. L.; Han, X. X.; Wang, T.; Guo, S. S.; Dong, T. Y.; Xu, J. C.; Anderson, G. J. et al. Targeted brain delivery of rabies virus glycoprotein 29-modified deferoxamine-loaded nanoparticles reverses functional deficits in parkinsonian mice. ACS Nano 2018, 12, 4123–4139.
Wei, Z. Y. D.; Shetty, A. K. Treating Parkinson’s disease by astrocyte reprogramming: Progress and challenges. Sci. Adv. 2021, 7, eabg3198.
Noble, E. E.; Wang, Z.; Liu, C. M.; Davis, E. A.; Suarez, A. N.; Stein, L. M.; Tsan, L.; Terrill, S. J.; Hsu, T. M.; Jung, A. H. et al. Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat. Commun. 2019, 10, 4923.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos. 22175085 and 21875101), National Key Research and Development Program of China (No. 2017YFA0701301), and State Key Laboratory of Analytical Chemistry for Life Science (No. SKLACLS2219).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yao, K., Gan, J., Zhao, D. et al. A core-satellite-like nanoassembly reverses a decisive tyrosine hydroxylase loss in degenerative dopaminergic neurons. Nano Res. 16, 9835–9847 (2023). https://doi.org/10.1007/s12274-023-5729-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5729-4