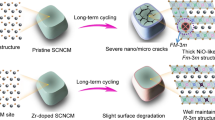
Abstract
The residual Li and Li+/Ni2+ cation mixing play essential roles in the electrochemical properties of Ni-rich cathodes. However, a general relationship between the residual Li conversion, cation mixing, and their effects on the Li+ kinetics and structural stability has yet to be established, due to the presence of cobalt in the cathode. Here, we explore the synergistic impact of the residual Li conversion and cation ordering on a Co-free Ni-rich cathode (i.e., LiNi0.95Mn0.05O2). It discloses that the rate capability is mainly affected by residual Li contents and operating voltage. Specifically, residual Li can be electrochemically converted to cathode electrolyte interphase (CEI) below 4.3 V, thus inducing high interphase resistance, and decomposes to produce CO2-dominated gas at 4.5 V, causing temporary enhancement of Li+ diffusivity but severe surface degradation during cycling. Moreover, the cycling performance of Co-free Ni-rich cathode is not only determined by Li+/Ni2+ cation-ordered superlattice, which enhances the structural stability as it functions as the pillar to impede lattice collapse at a highly charged state, but also by the robust CEI layers which protect the bulk from electrolyte attack under 4.3 V. These findings promote an in-depth understanding of residual Li conversion and Li+/Ni2+ cation ordering on Co-free Ni-rich cathode.

Similar content being viewed by others
References
Luo, D.; Fan, J. M.; Yao, Z.; **e, H. X.; Cui, J. X.; Yang, Y. J.; Ding, X. K.; Ji, J. P.; Wu, S. X.; Ling, M. et al. An almost full reversible lithium-rich cathode: Revealing the mechanism of high initial coulombic efficiency. J. Energy Chem. 2021, 62, 120–126.
Zou, Y. G.; Mao, H. C.; Meng, X. H.; Du, Y. H.; Sheng, H.; Yu, X. Q.; Shi, J. L.; Guo, Y. G. Mitigating the kinetic hindrance of single-crystalline Ni-rich cathode via surface gradient penetration of tantalum. Angew. Chem., Int. Ed. 2021, 60, 26535–26539.
Liu, Y. C.; Chen, Y. F.; Wang, J.; Wang, W.; Ding, Z. Y.; Li, L. Y.; Zhang, Y.; Deng, Y. D.; Wu, J. W.; Chen, Y. N. Hierarchical yolk—shell structured Li-rich cathode boosting cycling and voltage stabled LIBs. Nano Res. 2022, 15, 3178–3186.
Nam, G. W.; Park, N. Y.; Park, K. J.; Yang, J. H.; Liu, J.; Yoon, C. S.; Sun, Y. K. Capacity fading of Ni-rich NCA cathodes: Effect of microcracking extent. ACS Energy Lett. 2019, 4, 2995–3001.
You, B. Z.; Wang, Z. X.; Shen, F.; Chang, Y. J.; Peng, W. J.; Li, X. H.; Guo, H. J.; Hu, Q. Y.; Deng, C. W.; Yang, S. et al. Research progress of single-crystal nickel-rich cathode materials for lithium ion batteries. Small Methods 2021, 5, 2100234.
Du, Y. H.; Sheng, H.; Meng, X. H.; Zhang, X. D.; Zou, Y. G.; Liang, J. Y.; Fan, M.; Wang, F. Y.; Tang, J. L.; Cao, F. F. et al. Chemically converting residual lithium to a composite coating layer to enhance the rate capability and stability of single-crystalline Ni-rich cathodes. Nano Energy 2022, 94, 106901.
Wu, F.; Liu, N.; Chen, L.; Li, N.; Dong, J. Y.; Lu, Y.; Tan, G. Q.; Xu, M. Z.; Cao, D. Y.; Liu, Y. F. et al. The nature of irreversible phase transformation propagation in nickel-rich layered cathode for lithium-ion batteries. J. Energy Chem. 2021, 62, 351–358.
Zou, Y. H.; Yang, X. F.; Lv, C. X.; Liu, T. C.; **a, Y. Z.; Shang, L.; Waterhouse, G. I. N.; Yang, D. J.; Zhang, T. R. Multishelled Ni-rich Li(NixCoyMnz)O2 hollow fibers with low cation mixing as high-performance cathode materials for Li-ion batteries. Adv. Sci. 2017, 4, 1600262.
Liu, W.; Oh, P.; Liu, X. E.; Lee, M. J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem., Int. Ed. 2015, 54, 4440–4457.
Kim, U. H.; Park, G. T.; Son, B. K.; Nam, G. W.; Liu, J.; Kuo, L. Y.; Kaghazchi, P.; Yoon, C. S.; Sun, Y. K. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge. Nat. Energy 2020, 5, 860–869.
Li, M.; Lu, J. Cobalt in lithium-ion batteries: Replacements are sought for cobalt, a costly element used in lithium-ion battery cathodes. Science 2020, 367, 979–980.
Choi, J. U.; Voronina, N.; Sun, Y. K.; Myung, S. T. Recent progress and perspective of advanced high-energy Co-less Ni-rich cathodes for Li-ion batteries: Yesterday, today, and tomorrow. Adv. Energy Mater. 2020, 10, 2002027.
Liu, T. C.; Yu, L.; Liu, J. J.; Lu, J.; Bi, X. X.; Dai, A.; Li, M.; Li, M. F.; Hu, Z. X.; Ma, L. et al. Understanding Co roles towards develo** Co-free Ni-rich cathodes for rechargeable batteries. Nat. Energy 2021, 6, 277–286.
Cho, D. H.; Jo, C. H.; Cho, W.; Kim, Y. J.; Yashiro, H.; Sun, Y. K.; Myung, S. T. Effect of residual lithium compounds on layer Ni-rich Li[Ni0.7Mn0.3]O2. J. Electrochem. Soc. 2014, 161, A920–A926.
Zhang, S. S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2020, 24, 247–254.
Seong, W. M.; Cho, K. H.; Park, J. W.; Park, H.; Eum, D.; Lee, M. H.; Kim, I. S. S.; Lim, J.; Kang, K. Controlling residual lithium in high-nickel (> 90 %) lithium layered oxides for cathodes in lithium-ion batteries. Angew. Chem., Int. Ed. 2020, 59, 18662–18669.
Xu, G. L.; Liu, X.; Daali, A.; Amine, R.; Chen, Z. H.; Amine, K. Challenges and strategies to advance high-energy nickel-rich layered lithium transition metal oxide cathodes for harsh operation. Adv. Funct. Mater. 2020, 30, 2004748.
Zhang, J. T.; Tan, X. H.; Guo, L. M.; Jiang, Y.; Liu, S. N.; Wang, H. F.; Kang, X. H.; Chu, W. G. Controllable formation of lithium carbonate surface phase during synthesis of nickel-rich LiNi0.9Mn0.1O2 in air and its protection role in electrochemical reaction. J. Alloys Compd. 2019, 771, 42–50.
Li, L. J.; Chen, J. X.; Huang, H.; Tan, L.; Song, L. B.; Wu, H. H. Wang, C.; Zhao, Z. X.; Yi, H. L.; Duan, J. F. et al. Role of residual Li and oxygen vacancies in Ni-rich cathode materials. ACS Appl. Mater. Interfaces 2021, 13, 42554–42563.
Li, L. J.; Fu, L. Z.; Li, M.; Wang, C.; Zhao, Z. X.; **e, S. C.; Lin, H. C.; Wu, X. W.; Liu, H. D.; Zhang, L. et al. B-doped and La4NiLiO8-coated Ni-rich cathode with enhanced structural and interfacial stability for lithium-ion batteries. J. Energy Chem. 2022, 71, 588–594.
Ryu, H. H.; Park, N. Y.; Yoon, D. R.; Kim, U. H.; Yoon, C. S.; Sun, Y. K. New class of Ni-rich cathode materials Li[NixCoyB1−x−y]O2 for next lithium batteries. Adv. Energy Mater. 2020, 10, 2000495.
Wang, D. W.; Kou, R. H.; Ren, Y.; Sun, C. J.; Zhao, H.; Zhang, M. J.; Li, Y.; Huq, A.; Ko, J. Y. P.; Pan, F. et al. Synthetic control of kinetic reaction pathway and cationic ordering in high-Ni layered oxide cathodes. Adv. Mater. 2017, 29, 1606715.
Wang, S. N.; Hua, W. B.; Missyul, A.; Darma, M. S. D.; Tayal, A.; Indris, S.; Ehrenberg, H.; Liu, L. J.; Knapp, M. Kinetic control of long-range cationic ordering in the synthesis of layered Ni-rich oxides. Adv. Funct. Mater. 2021, 31, 2009949.
Seong, W. M.; Kim, Y.; Manthiram, A. Impact of residual lithium on the adoption of high-nickel layered oxide cathodes for lithium-ion batteries. Chem. Mater. 2020, 32, 9479–9489.
Kim, Y.; Park, H.; Warner, J. H.; Manthiram, A. Unraveling the intricacies of residual lithium in high-Ni cathodes for lithium-ion batteries. ACS Energy Lett. 2021, 6, 941–948.
Yang, H. P.; Wu, H. H.; Ge, M. Y.; Li, L. J.; Yuan, Y. F.; Yao, Q.; Chen, J.; **a, L. F.; Zheng, J. M.; Chen, Z. Y. et al. Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium-ion batteries. Adv. Funct. Mater. 2019, 29, 1808825.
Ke, C. Z.; Liu, F.; Zheng, Z. M.; Zhang, H. H.; Cai, M. T.; Li, M.; Yan, Q. Z.; Chen, H. X.; Zhang, Q. B. Boosting lithium storage performance of Si nanoparticles via thin carbon and nitrogen/phosphorus co-doped two-dimensional carbon sheet dual encapsulation. Rare Met. 2021, 40, 1347–1356.
Liu, Y.; Tang, L. B.; Wei, H. X.; Zhang, X. H.; He, Z. J.; Li, Y. J.; Zheng, J. C. Enhancement on structural stability of Ni-rich cathode materials by in-situ fabricating dual-modified layer for lithium-ion batteries. Nano Energy 2019, 65, 104043.
Fan, X. M.; Ou, X.; Zhao, W. G.; Liu, Y.; Zhang, B.; Zhang, J. F.; Zou, L. F.; Seidl, L.; Li, Y. Z.; Hu, G. R. et al. In situ inorganic conductive network formation in high-voltage single-crystal Ni-rich cathodes. Nat. Commun. 2021, 12, 5320.
Feng, X. Y.; Wu, H. H.; Gao, B.; Świętosławski, M.; He, X.; Zhang, Q. B. Lithiophilic N-doped carbon bowls induced Li deposition in layered graphene film for advanced lithium metal batteries. Nano Res. 2022, 15, 352–360.
Cai, M. T.; Zhang, H. H.; Zhang, Y. G.; **ao, B. S.; Wang, L.; Li, M.; Wu, Y.; Sa, B. S.; Liao, H. G.; Zhang, L. et al. Boosting the potassium-ion storage performance enabled by engineering of hierarchical MoSSe nanosheets modified with carbon on porous carbon sphere. Sci. Bull. 2022, 67, 933–945.
Sheng, H.; Meng, X. H.; **ao, D. D.; Fan, M.; Chen, W. P.; Wan, J.; Tang, J. L.; Zou, Y. G.; Wang, F. Y.; Wen, R. et al. An air-stable high-nickel cathode with reinforced electrochemical performance enabled by convertible amorphous Li2CO3 modification. Adv. Mater. 2022, 34, 2108947.
Liu, W.; Li, J. X.; Li, W. T.; Xu, H. Y.; Zhang, C.; Qiu, X. P. Inhibition of transition metals dissolution in cobalt-free cathode with ultrathin robust interphase in concentrated electrolyte. Nat. Commun. 2020, 11, 3629.
Heenan, T. M. M.; Wade, A.; Tan, C.; Parker, J. E.; Matras, D.; Leach, A. S.; Robinson, J. B.; Llewellyn, A.; Dimitrijevic, A.; Jervis, R. et al. Identifying the origins of microstructural defects such as cracking within Ni-rich NMC811 cathode particles for lithium-ion batteries. Adv. Energy Mater. 2020, 10, 2002655.
Sun, H. H.; Manthiram, A. Impact of microcrack generation and surface degradation on a nickel-rich layered Li[Ni0.9Co0.05Mn0.05]O2 cathode for lithium-ion batteries. Chem. Mater. 2017, 29, 8486–8493.
Li, H. Y.; Liu, A.; Zhang, N.; Wang, Y. Q.; Yin, S.; Wu, H. H.; Dahn, J. R. An unavoidable challenge for Ni-rich positive electrode materials for lithium-ion batteries. Chem. Mater. 2019, 31, 7574–7583.
Aishova, A.; Park, G. T.; Yoon, C. S.; Sun, Y. K. Cobalt-free high-capacity Ni-rich layered Li[Ni0.9Mn0.1]O2 cathode. Adv. Energy Mater. 2020, 10, 1903179.
Kim, U. H.; Park, G. T.; Conlin, P.; Ashburn, N.; Cho, K.; Yu, Y. S.; Shapiro, D. A.; Maglia, F.; Kim, S. J.; Lamp, P. et al. Cation ordered Ni-rich layered cathode for ultra-long battery life. Energy Environ. Sci. 2021, 14, 1573–1583.
Yoon, C. S.; Choi, M. J.; Jun, D. W.; Zhang, Q.; Kaghazchi, P.; Kim, K. H.; Sun, Y. K. Cation ordering of Zr-doped LiNiO2 cathode for lithium-ion batteries. Chem. Mater. 2018, 30, 1808–1814.
Acknowledgement
This work was financially supported by the National Natural Science Foundation of China (No. 51774051), the Science and Technology Planning Project of Hunan Province (No. 2019RS2034), the Hunan High-tech Industry Science and Technology Innovation Leading Plan (No. 2020GK2072), and the Changsha City Fund for Distinguished and Innovative Young Scholars (No. KQ1707014).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2022_4889_MOESM1_ESM.pdf
Unveiling the impact of residual Li conversion and cation ordering on electrochemical performance of Co-free Ni-rich cathodes
Rights and permissions
About this article
Cite this article
Wang, C., Tan, L., Yi, H. et al. Unveiling the impact of residual Li conversion and cation ordering on electrochemical performance of Co-free Ni-rich cathodes. Nano Res. 15, 9038–9046 (2022). https://doi.org/10.1007/s12274-022-4889-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4889-0