Abstract
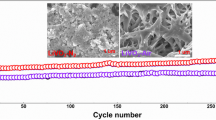
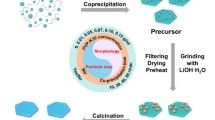
Oxygen-deficient LiV3O8 is considered as one of the promising cathode materials for lithium ion batteries (LIBs) because of its high cycling stability and rate capability. However, it is very difficult to control and study the content and position of V4+ and oxygen vacancies in LiV3O8, and therefore the mechanism of improving electrochemical performance of LiV3O8 is still unclear. Herein, we developed four LiV3O8 nanosheets with different V4+ and oxygen vacancy contents and positions. The physicochemical and lithium storage properties indicate that the V4+ and oxygen vacancies in the surface layer increase the contribution of pseudocapacitive lithium storage on the nanosheet surface. The V4+ and oxygen vacancies in the lattice improve the electrical conductivity of LiV3O8, and enhance the phase transformation and lithium ion diffusion rates. By adjusting the content of V4+ and oxygen vacancies, we obtained an oxygen-deficient LiV3O8 nanosheet which maintained more than 93% of the initial reversible capacity after 300 cycles at 5,000 mA·g−1. The V4+ and oxygen vacancies play an important role in improving the stability and rapidity of lithium storage. This work is helpful to understand the stable and fast lithium storage mechanism of oxygen-deficient LiV3O8, and might lay a foundation for further studies of other oxygen-deficient metal oxide electrodes for long-life and high-power LIBs.

Similar content being viewed by others
References
Zhang, Y. P.; Wang, L. L.; Xu, H.; Cao, J. M.; Chen, D.; Han, W. 3D chemical cross-linking structure of black phosphorus@CNTs hybrid as a promising anode material for lithium ion batteries. Adv. Funct. Mater. 2020, 30, 1909372.
Wang, H. W.; Fu, J. Z.; Wang, C.; Wang, J. Y.; Yang, A. K.; Li, C. C.; Sun, Q. F.; Cui, Y.; Li, H. Q. A binder-free high silicon content flexible anode for Li-ion batteries. Energy Environ. Sci. 2020, 13, 848–858.
Deng, Y. L.; Ma, L. L.; Li, T. H.; Li, J. Y.; Yuan, C. Life cycle assessment of silicon-nanotube-based lithium ion battery for electric vehicles. ACS Sustain. Chem. Eng. 2019, 7, 599–610.
Song, Z. H.; Feng, K.; Zhang, H. Z.; Guo, P.; Jiang, L. H.; Wang, Q. S.; Zhang, H. M.; Li, X. F. “Giving comes before receiving”: High performance wide temperature range Li-ion battery with Li5 V2 (PO4)3 as both cathode material and extra Li donor. Nano Energy 2019, 66, 104175.
Wang, F. X.; Wu, X. W.; Li, C. Y.; Zhu, Y. S.; Fu, L. J.; Wu, Y. P.; Liu, X. Nanostructured positive electrode materials for post-lithium ion batteries. Energy Environ. Sci. 2016, 9, 3570–3611.
Guo, B. K.; Yu, X. Q.; Sun, X. G.; Chi, M. F.; Qiao, Z. A.; Liu, J.; Hu, Y. S.; Yang, X. Q.; Goodenough, J. B.; Dai, S. A long-life lithium-ion battery with a highly porous TiNb2O7 anode for large-scale electrical energy storage. Energy Environ. Sci. 2014, 7, 2220–2226.
Luan, Y. T.; Yin, J. L.; Zhu, K.; Cheng, K.; Yan, J.; Ye, K.; Wang, G. L.; Cao, D. X. Arc-discharge production of high-quality fluorine-modified graphene as anode for Li-ion battery. Chem. Eng. J. 2020, 392, 123668.
Li, X. D.; Wu, G. X.; Liu, X.; Li, W.; Li, M. C. Orderly integration of porous TiO2 (B) nanosheets into bunchy hierarchical structure for high-rate and ultralong-lifespan lithium-ion batteries. Nano Energy 2017, 37, 1–8.
Yu, X. L.; Deng, J. J.; Yang, X.; Li, J.; Huang, Z. H.; Li, B. H.; Kang, F. Y. A dual-carbon-anchoring strategy to fabricate flexible LiMn2O4 cathode for advanced lithium-ion batteries with high areal capacity. Nano Energy 2020, 67, 104256.
Hu, L. H.; Wu, F. Y.; Lin, C. T.; Khlobystov, A. N.; Li, L. J. Graphene-modified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687.
Park, K.; Yu, B. C.; Jung, J. W.; Li, Y. T.; Zhou, W. D.; Gao, H. C.; Son, S.; Goodenough, J. B. Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: Interface between LiCoO2 and garnet-Li7 La3 Zr2O12. Chem. Mater. 2016, 28, 8051–8059.
Thamodaran, P.; Kesavan, T.; Vivekanantha, M.; Senthilkumar, B.; Barpanda, P.; Sasidharan, M. Operando structural and electrochemical investigation of Li1.5 V3O8 nanorods in Li-ion batteries. ACS Appl. Energy Mater. 2019, 2, 852–859.
Zhang, Q.; Brady, A. B.; Pelliccione, C. J.; Bock, D. C.; Bruck, A. M.; Li, J.; Sarbada, V.; Hull, R.; Stach, E. A.; Takeuchi, K. J. et al. Investigation of structural evolution of Li1.1 V3O8 by in situ X-ray diffraction and density functional theory calculations. Chem. Mater. 2017, 29, 2364–2373.
Bae, K. Y.; Jung, Y. H.; Cho, S. H.; Kim, B. H.; Yoon, W. Y. Design and analysis of an optimal cathode for Li-LiV3O8 secondary cells. J. Alloys Compd. 2019, 784, 704–711.
de Picciotto, L. A.; Adendorff, K. T.; Liles, D. C.; Thackeray, M. M. Structural characterization of Li1+x V3O8 insertion electrodes by single-crystal X-ray diffraction. Solid State Ionics 1993, 62, 297–307.
Wadsley, A. D. Crystal chemistry of non-stoichiometric pentavalent vandadium oxides: Crystal structure of Li1+x V3O8. Acta Crystallogr. 1957, 10, 261–267.
Wang, L. P.; Deng, L. B.; Li, Y. L.; Ren, X. Z.; Mi, H. W.; Sun, L. N.; Zhang, P. X.; Gao, Y. Nb5+ doped LiV3O8 nanorods with extraordinary rate performance and cycling stability as cathodes for lithium-ion batteries. Electrochim. Acta 2018, 284, 366–375.
Partheeban, T.; Sasidharan, M. Template-free synthesis of LiV3O8 hollow microspheres as positive electrode for Li-ion batteries. J. Mater. Sci. 2020, 55, 2155–2165.
Liu, H. M.; Wang, Y. G.; Wang, K. X.; Wang, Y. R.; Zhou, H. S. Synthesis and electrochemical properties of single-crystalline LiV3O8 nanorods as cathode materials for rechargeable lithium batteries. J. Power Sources 2009, 192, 668–673.
Kawakita, J.; Kato, T.; Katayama, Y.; Miura, T.; Kishi, T. Lithium insertion behaviour of Li1+x V3O8 with different degrees of crystallinity. J. Power Sources 1999, 81–82, 448–453.
Wu, Z. J.; Zhou, Y. Effect of Ce-do** on the structure and electrochemical performance of lithium trivanadate prepared by a citrate sol-gel method. J. Power Sources 2012, 199, 300–307.
Song, H. Q.; Luo, M. S.; Wang, A. M. High rate and stable Li-ion insertion in oxygen-deficient LiV3O8 nanosheets as a cathode material for lithium-ion battery. ACS Appl. Mater. Interfaces 2017, 9, 2875–2882.
Song, H. Q.; Liu, Y. G.; Zhang, C. P.; Liu, C. F.; Cao, G. Z. Mo-doped LiV3O8 nanorod-assembled nanosheets as a high performance cathode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 3547–3558.
Zheng, J. Q.; Zhang, Y. F.; Wang, N. N.; Zhao, Y. F.; Tian, F. P.; Meng, C. G. Facile synthesis and characterization of LiV3O8 with sheet-like morphology for high-performance supercapacitors. Mater. Lett. 2016, 171, 240–243.
Wang, Z. K.; Shu, J.; Zhu, Q. C.; Cao, B. Y.; Chen, H.; Wu, X. Y.; Bartlett, B. M.; Wang, K. X.; Chen, J. S. Graphene-nanosheet-wrapped LiV3O8 nanocomposites as high performance cathode materials for rechargeable lithium-ion batteries. J. Power Sources 2016, 307, 426–434.
Liu, J.; Liu, W.; Wan, Y. L.; Ji, S. M.; Wang, J. B.; Zhou, Y. C. Facile synthesis of layered LiV3O8 hollow nanospheres as superior cathode materials for high-rate Li-ion batteries. RSC Adv. 2012, 2, 10470–10474.
Wang, H. Y.; Ren, Y.; Wang, Y.; Wang, W. J.; Liu, S. Q. Synthesis of LiV3O8 nanosheets as a high-rate cathode material for rechargeable lithium batteries. CrystEngComm 2012, 14, 2831–2836.
Mo, R. W.; Du, Y.; Zhang, N. Q.; Rooney, D.; Sun, K. N. In situ synthesis of LiV3O8 nanorods on graphene as high rate-performance cathode materials for rechargeable lithium batteries. Chem. Commun. 2013, 49, 9143–9145.
Sun, D.; **, G. H.; Wang, H. Y.; Huang, X. B.; Ren, Y.; Jiang, J. C.; He, H. N.; Tang, Y. G. Lix V2O5/LiV3O8 nanoflakes with significantly improved electrochemical performance for Li-ion batteries. J. Mater. Chem. A 2014, 2, 8009–8016.
Zhang, Z.; Tan, X.; Yu, T.; Jia, L. X.; Huang, X. Time-dependent formation of oxygen vacancies in black TiO2 nanotube arrays and the effect on photoelectrocatalytic and photoelectrochemical properties. Int. J. Hydrogen Energy 2016, 41, 11634–11643.
Li, Y. W.; Yao, J. H.; Uchaker, E.; Zhang, M.; Tian, J. J.; Liu, X. Y.; Cao, G. Z. Sn-doped V2O5 film with enhanced lithium-ion storage performance. J. Phys. Chem. C 2013, 117, 23507–23514.
Yan, Y.; Hao, B.; Wang, D.; Chen, G.; Markweg, E.; Albrecht, A.; Schaaf, P. Understanding the fast lithium storage performance of hydrogenated TiO2 nanoparticles. J. Mater. Chem. A 2013, 7, 14507–14513.
Liu, D. W.; Liu, Y. Y.; Pan, A. Q.; Nagle, K. P.; Seidler, G. T.; Jeong, Y. H.; Cao, G. Z. Enhanced lithium-ion intercalation properties of V2O5 xerogel electrodes with surface defects. J. Phys. Chem. C 2011, 115, 4959–4965.
Sakunthala, A.; Reddy, M. V.; Selvasekarapandian, S.; Chowdari, B. V. R.; Selvin, P. C. Preparation, characterization, and electrochemical performance of lithium trivanadate rods by a surfactant-assisted polymer precursor method for lithium batteries. J. Phys. Chem. C 2010, 114, 8099–8107.
Wang, Y. L.; Xu, X. Y.; Cao, C. B.; Shi, C.; Mo, W.; Zhu, H. S. Synthesis and performance of Li1.5 V3O8 nanosheets as a cathode material for high-rate lithium-ion batteries. J. Power Sources 2013, 242, 230–235.
Guo, H. P.; Liu, L.; Shu, H. B.; Yang, X. K.; Yang, Z. H.; Zhou, M.; Tan, J. L.; Yan, Z. C.; Hu, H.; Wang, X. Y. Synthesis and electrochemical performance of LiV3O8/polythiophene composite as cathode materials for lithium ion batteries. J. Power Sources 2014, 247, 117–126.
Jouanneau, S.; Le Gal La Salle, A.; Verbaere, A.; Deschamps, M.; Lascaud, S.; Guyomard, D. Influence of the morphology on the Li insertion properties of Li1.1 V3O8. J. Mater. Chem. 2003, 13, 921–927.
Jouanneau, S.; Le Gal La Salle, A.; Verbaere, A.; Guyomard, D. The origin of capacity fading upon lithium cycling in Li1.1 V3O8. J. Electrochem. Soc. 2005, 152, A1660–A1667.
Tatara, R.; Karayaylali, P.; Yu, Y.; Zhang, Y. R.; Giordano, L.; Maglia, F.; Jung, R.; Schmidt, J. P.; Lund, I.; Shao-Horn, Y. The effect of electrode-electrolyte interface on the electrochemical impedance spectra for positive electrode in Li-ion battery. J. Electrochem. Soc. 2018, 166, A5090–A5098.
Conway, B. E.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 1997, 66, 1–14.
Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931.
Qiu, D. P.; Kang, C. H.; Li, M.; Wei, J. Y.; Hou, Z. W.; Wang, F.; Yang, R. Biomass-derived mesopore-dominant hierarchical porous carbon enabling ultra-efficient lithium ion storage. Carbon 2020, 162, 595–603.
Augustyn, V.; Come, J.; Lowe, M. A.; Kim, J. W.; Taberna, P. L.; Tolbert, S. H.; Abruña, H. D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522.
Li, H. Y.; Cheng, Z.; Zhang, Q.; Natan, A.; Yang, Y.; Cao, D. X.; Zhu, H. J. Bacterial-derived, compressible, and hierarchical porous carbon for high-performance potassium-ion batteries. Nano Lett. 2018, 18, 7407–7413.
Li, Z.; Cao, L. J.; Chen, W.; Huang, Z. C.; Liu, H. Mesh-like carbon nanosheets with high-level nitrogen do** for high-energy dual-carbon lithium-ion capacitors. Small 2019, 15, 1805173.
Sun, W. W.; Tao, X. C.; Du, P. P.; Wang, Y. Carbon-coated mixed-metal sulfide hierarchical structure: MOF-derived synthesis and lithium-storage performances. Chem. Eng. J. 2019, 366, 622–630.
Acknowledgements
The authors thank for the financial support of Bei**g Natural Science Foundation (No. 2182015) and the National Natural Science Foundation of China (No. 21805012).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Notes
The authors declare no competing financial interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Song, H., Liu, F. & Luo, M. Insights into the stable and fast lithium storage performance of oxygen-deficient LiV3O8 nanosheets. Nano Res. 14, 814–822 (2021). https://doi.org/10.1007/s12274-020-3118-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-3118-9