Abstract
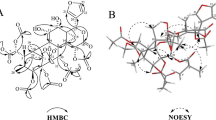
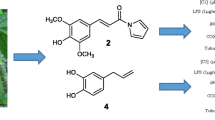
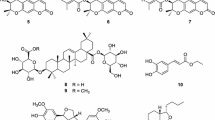
Pongamia pinnata (Linn.) Pierre has anti-inflammatory activity and could significantly decrease serum tumor necrosis factor-α and IL-10 in arthritic rats. Previous research indicated the typical chemical constituent in P. pinnata is furanoflavone. Guided by anti-inflammatory active assay and UPLC-HRESIMS chromatography, 22 compounds were isolated from the ethanol extract of P. pinnata seedpods. One novel furanoflavone, 4′-hydroxypinnatin, was elucidated by HRESIMS, 1D- and 2D-NMR spectra. The 21 known compounds, including 9 furanoflavone, were identified by comparing their NMR data with the previous data in reference. In the known compounds, 5 were isolated for the first time from the species. The anti-inflammatory activities were assayed by assessing LPS-induced NO production in BV-2 cells. 12 compounds can inhibit the production of NO without cytotoxicity at concentration of 50 μM. Among them, compounds 4 can significantly inhibit the production of NO, with the IC50 value of 31.36 μM.




Similar content being viewed by others
References
Ahmad G, Yadav PP, Maurya R (2004) Furanoflavonoid glycosides from Pongamia pinnata fruits. Phytochemistry 65:921–924
Al Muqarrabun LM, Ahmat N, Ruzaina SA, Ismail NH, Sahidin I (2013) Medicinal uses, phytochemistry and pharmacology of Pongamia pinnata (L.) Pierre: a review. J Ethnopharmacol 150:395–420
Bezuidenhout SC, Bezuidenhoudt BCB, Brandt EV, Ferreira D (1988) Oligomeric isoflavonoids. Part 2. Structure and synthesis of xanthocercin A and B, the first isoflavono-lignoids. J Chem Soc Perkin Trans 1:1237–1241
Bose M, Chakraborty M, Bhattacharya S, Bhattacharjee P, Mandal S, Kar M, Mishra R (2014) Suppression of NF-kappaB p65 nuclear translocation and tumor necrosis factor-alpha by Pongamia pinnata seed extract in adjuvant-induced arthritis. J Immunotoxicol 11:222–230
Dong CX, Wu KS, Shi SP, Tu PF (2006) Flavanoids from Clematis hexapetala. J Chin Pharm Sci 15:15
Ekdahl CT, Kokaia Z, Lindvall O (2009) Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158:1021–1029
Khandelwal PJ, Herman AM, Moussa CE (2011) Inflammation in the early stages of neurodegenerative pathology. J Neuroimmunol 238:1–11
Li LY, Li X, Shi C, Deng Z, Lin W (2008) Studies on chemical constituents of semi-mangrove plant Pongamia pinnata. Chin J Mar Drugs 27:18
Li J, Jiang Z, Li X, Hou Y, Liu F, Li N, Liu X, Yang L (2015) Natural therapeutic agents for neurodegenerative diseases from a traditional herbal medicine Pongamia pinnata (L.) Pierre. Bioorg Med Chem Lett 25:53–58
Ma X, Wang L, Guo Y, Guo S (2005) A new isoflavone from Huoxue Yiqi Tang. Chin J Chin Mater Med 30:1159
Marzouk MSA, Ibrahim MT, El-Gindi OR, Abou Bakr MS (2008) Isoflavonoid glycosides and rotenoids from Pongamia pinnata leaves. Z Naturforschung C 63:1–7
Muthu C, Ayyanar M, Raja N, Ignacimuthu S (2006) Medicinal plants used by traditional healers in Kancheepuram district of Tamil Nadu, India. J Ethnobiol Ethnomed 2:43
Pathak VP, Khanna RN (1981) Synthesis of gamatin, naturally occurring linear furanoflavone. Indian J Chem 20B:622
Polazzi E, Monti B (2010) Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol 92:293–315
Prashant A, Krupadanam GLD (1993) Dehydro-6-hydroxyrotenoid and lupenone form tephrosia villosa. Phytochemistry 32:484
Sang S, Lapsley K, Jeong WS, Lachance PA, Ho CT, Rosen RT (2002) Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batsch). J Agric Food Chem 50:2459
Singh RK, Joshi VK, Goel RK, Gambhir SS, Acharya SB (1996) Pharmacological actions of Pongamia pinnata seeds—a preliminary study. Indian J Exp Biol 34:1204–1207
Talapatra SK, Mallik AK, Talapatra B (1980) Pongaglabol, a new hydroxyfuranflavone and aurantiamide acetate, a dipeptide from the flowers of Pongamia glabra. Phytochemistry 19:1199
Talapatra SK, Mallik AK, Talapatra B (1982) Isopongaglabol and 6-methoxyisopongaglabol, two new hydroxyfuranoflavones from Pongamia glabra. Phytochemistry 21:761
Tanaka T, Iinuma M, Yuki K, Fujii Y, Mizuno M (1992) Flavonoids in root bark of Pongamia-Pinnata. Phytochemistry 31:993–998
Togna AR, Latina V, Trefiletti G, Guiso M, Moschini S, Togna GI (2013) 1-Phenil-6,7-dihydroxy-isochroman inhibits inflammatory activation of microglia. Brain Res Bull 95:33–39
Wang X, Che Q, Li Y, He Y (1999) A study on chemical constituents in seeds of Crataegus pinnatifida Bge. var. major N.E.Br. Chin J Chin Mater Med 24:739
Wang M, T-F Ji, Yang J, Su Y (2009) Studies on the chemical constituents of Phellodendron chinense. Chin Med Mater 32:208
Yi YH, Li L, Lin HW, Li WL, Yao XS, Tang HF, Xu QZ, Zou ZR, Sun P, Zhang SY, Liu BS (2001) Studies on antineoplastic constituents from marine organisms in south China sea. Acad J Second Mil Med Univ 23:236
Yu J, Lin Z, Wang D, Sun H (1994) A sesquiterpene and other constituents from Erigeron breviscapus. Phytochemistry 36:717
Zou ZR, Yi YH, Yao X-S, Jun DL, Zhou DZ, Zhang SY (2004) Studies on chemical constituents of Acaudina molpadioides semper. Chin J Nat Med 2:348
Acknowledgements
This work was supported partially by National Natural Science Foundation of China (Nos. 81303183, 81303182 and U1403102) and by the Project of Science and Technology Correspondent of Tian** (No. 15JCTPJC60000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared no conflict of interest.
Additional information
Hong Guo and Zisong Bai have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Guo, H., Bai, Z., Xu, Y. et al. Anti-inflammation compounds from the seedpods of Pongamia pinnata (L.) Pierre guided by the bioactivity and UPLC-HRESIMS. Arch. Pharm. Res. 40, 818–824 (2017). https://doi.org/10.1007/s12272-017-0913-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0913-2