Abstract
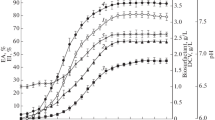
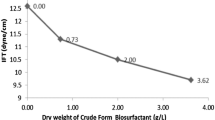
Biosurfactants are amphiphilic compounds produced by several microorganisms that reduce the surface tension. Low toxicity, optimal activity in extreme conditions, biodegradability and production from several wastes are main advantages of biosurfactants as compared to synthetic surfactants. Production of biosurfactant by a white rot fungus Pleurotus djamor on sunflower seed shell in solid-state fermentation was determined by emulsification indexes, oil spreading activity and surface tension (28.82 ± 0.3mN/m) measurement. The critical micelle concentration was detected as 0.964 ± 0.09 mg/mL. Also, the chemical and physicochemical properties of the biosurfactant produced were investigated. Considering the results of the chemical contents analysis, HPLC, FT-IR and 1H-NMR, it can be concluded that the produced biosurfactant has a complex structure. Besides, resistance of its activity to environmental factors such as temperature, pH and salt concentration, as well as its thermal stability, were investigated. Additionally, the produced biosurfactant formed stabile emulsions with different hydrocarbons. Lastly, the performance of removing waste frying oil from contaminated sand of produced biosurfactant was detected as 76.57 ± 6%. Owing to its high emulsification capacity, low surface tension and critical micelle concentration, the biosurfactant, shows great potential for use in hydrocarbon removal applications.
Similar content being viewed by others
References
Pacwa-Płociniczak, M., G. A. Płaza, Z. Piotrowska-Seget, and S. S. Cameotra (2011) Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 12: 633–654.
Nitschke, M. and S. G. V. A. O. Costa (2007) Biosurfactants in food industry. Trends Food Sci. Tech. 18: 252–259.
Muthusamy, K., S. Gopalakrishnan, T. K. Ravi, and P. Sivachidambaram (2008) Biosurfactants: properties, commercial production and application. Curr. Sci. 94: 736–747.
Saharan, B. S., R. K. Sahu, and D. Sharma (2011) A review on biosurfactants: Fermentation, current developments and perspectives. Gen. Eng. Biotech. J. 29: 1–39.
Makkar, R. S., S. S. Cameotra, and I. M. Banat (2011) Advances in utilization of renewable substrates for biosurfactant production. AMB Exp. 1: 1–19.
Pandey, A. (2003) Solid-state fermentation. Biochem. Eng. J. 13: 81–84.
Stajic, M., L. Persky, D. Friesem, and Y. Hadar (2006) Effect of different carbon and nitrogen sources on laccase and peroxidases production by selected Pleurotus species. Enz. Microb. Tech. 38: 65–73.
Velioglu, Z. and R. Ozturk Urek (2015) Optimization of cultural conditions for biosurfactant production by Pleurotus djamor in solid state fermentation. J. Biosci. Bioeng. 120: 526–531.
Bazalel, L., Y. Hadar, and C. Cerniglia (1997) Enzymatic mechanisms involved in phenanthrene degradation by the white rot fungus Pleurotus ostreatus. Appl. Environ. Microb. 63: 2495–2501.
Velioglu, Z. and R. Ozturk Urek (2014) Concurrent biosurfactant and ligninolytic enzyme production by Pleurotus spp. in solidstate fermentation. Appl. Biochem. Biotechnol. 174: 1354–1364.
Velioglu, Z. and R. Ozturk Urek (2015) Biosurfactant production by Pleurotus ostreatus in submerged and solid-state fermentation systems. Turk. J. Bio. 39: 160–166.
Pinto, M. H., R. G. Martins, and J. A. V. Costa (2009) Bacteria biosurfactants production kinetic evaluation. Química. Nova. 32: 2104–2108.
Youssef, N. H., K. E. Duncan, D. P. Nagle, and K. N. Savage (2004) Comparison of methods to detect biosurfactant production by diverse microorganisms. J. Microbiol. Meth. 56: 339–347.
Yin, H., J. Qiang, Y. Jia, and J. Ye (2009) Characteristics of bio-surfactant produced by Pseudomonas aeruginosa S6 isolated from oil-containing wastewater. Proc. Biochem. 44: 302–308.
Chander, C. R., T. Lohitnath, D. J. Mukesh Kumar, and P. T. Kalaichelvan (2012) Production and characterization of biosurfactant from Bacillus subtilis MTCC441 and its evaluation to use as bioemulsifier for food bio-preservative. Adv. Appl. Sci. Res. 3: 1827–1831.
Bradford, M. M. (1976) A rapid and sensitive method for the quantitation of microorganisms qualities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254
Miller, G. L. (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31: 426–428.
Dubois, M., K. A. Gilles, J. K. Hamilton, P. Rebers, and F. Smith (1956) Colorimetric method for determination of sugars and related substances. Anal. Chem. 28: 350–356.
Weatherburn, M. W. (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39: 971–974.
Mishra, S. K., W. I. Suh, W. Farooq, and M. Moon (2014) Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 155: 330–333.
Chandrasekaran, E. V. and J. N. Bemiller (1980) Constituent analysis of glycosaminoglycans. pp. 89–96. In: R. L. Whistler, and M. L. Wolfrom (eds.). Methods in carbohydrate chemistry. Academic Press, NY, USA.
Wie, Y. H. and I. M. Chu (2002) Mn2+ improves surfactin production by Bacillus subtilis. Biotechnol. Lett. 24: 479–482.
Abouseoud, M., R. Maachi, A. Amrane, S. Boudergua, and A. Nabi (2008) Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalinat. 223: 143–151.
Jain, R. M., K. Mody, A. Mishra, and B. Jha (2012a) Isolation and structural characterization of biosurfactant produced by an alkaliphilic bacterium Cronobacter sakazakii isolated from oil contaminated wastewater. Carbohyd. Polym. 87: 2320–2326.
Jain, R. M., K. Mody, A. Mishra, and B. Jha (2012b) Physicochemical characterization of biosurfactant and its potential to remove oil from soil and cotton cloth. Carbohyd. Polym. 89: 1110–1116.
Latha, R. and R. Kalaivani (2012) Bacterial degradation of crude oil by gravimetric analysis. Adv. Appl. Sci. Res. 3: 2789–2795.
Sarubbo, L. A., C. B. Farias, and G. M. Campos-Takaki (2007) Co-utilization of canola oil and glucose on the production of a surfactant by Candida lipolytica. Curr. Microbiol. 54: 68–73.
Benincasa, M., A. Abalos, I. Oliveira, and A. Manresa (2004) Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. A Van. Leeuw. J. Microb. 85: 1–8.
Abalos, A., A. Pinazo, M. R. Infante, and M. Casals (2001) Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir. 17: 1367–1371.
Luna, J. M. D., L. Sarubbo, and G. M. D. Campos-Takaki (2009) A new biosurfactant produced by Candida glabrata UCP 1002: Characteristics of stability and application in oil recovery. Braz. Arch. Biol. Techn. 52: 785–793.
Chooklin, C. S., S. Maneerat, and A. Saimmai (2014) Utilization of banana peel as a novel substrate for biosurfactant production by Halobacteriaceae archaeon AS65. Appl. Biochem. Biotechnol. 173: 624–645.
Gudiña, E. J., J. A. Teixeira, and L. R. Rodrigues (2010) Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloid. Surf. B. 76: 298–304.
Rochae Silva, N. M. P., R. D. Rufino, J. M. Luna, V. A. Santos, and L. A. Sarubbo (2014) Screening of Pseudomonas species for biosurfactant production using low-cost substrates. Biocatal. Agr. Biotechnol. 3: 132–139.
Markande, A. R., S. R. Acharya, and A. S. Nerurkar (2013) Physicochemical characterization of a thermostable glycoprotein bioemulsifier from Solibacillus silvestris AM1. Proc. Biochem. 48: 1800–1808.
Rodrigues, L., I. M. Banat, J. Teixeira, and R. Oliveira (2006) Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemoth. 57: 609–618.
Abbasi, H., M. M. Hamedi, T. B. Lotfabad, and H. S. Zahiri (2012) Biosurfactant-producing bacterium, Pseudomonas aeruginosa MA01 isolated from spoiled apples: Physicochemical and structural characteristics of isolated biosurfactant. J. Biosci. Bioeng. 113: 211–219.
Pereira, J. F., E. J. Gudiña, R. Costa, and R. Vitorino (2013) Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 111: 259–268.
Nikiforova, S. V., N. N. Pozdnyakova, and O. V. Turkovskaya (2009) Emulsifying agent production during PAHs degradation by the white rot fungus Pleurotus ostreatus D1. Curr. Microbiol. 58: 554–558.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Velioglu, Z., Urek, R.O. Physicochemical and structural characterization of biosurfactant produced by Pleurotus djamor in solid-state fermentation. Biotechnol Bioproc E 21, 430–438 (2016). https://doi.org/10.1007/s12257-016-0139-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-016-0139-z