Abstract
Objective
The aim of the present study is to modify the release profile of oxcarbazepine by formulating it as a bilayer tablet using direct compression method with dual compression.
Methods
In the bilayer system, the immediate release layer was formulated using four types of disintegrants, sodium starch glycolate (SSG), croscarmellose sodium (CCS), cross-linked polyvinylpyrrollidone (crospovidone), and starch. It was found that immediate release layer containing sodium starch glycolate gave faster disintegration time. Therefore, sodium starch glycolate was utilized in the preparation of bilayer tablets in this study. The sustained release layer was prepared utilizing three hydrophobic polymers, two of them are acrylics (Eudragit RS®, Eudragit RL®) and the third one is ethyl cellulose. The prepared bilayer tablets were evaluated for hardness, friability, weight variation, content uniformity, and dissolution profile.
Results
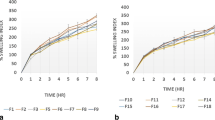
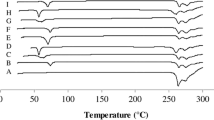
The results showed that combination of Eudragit RL® with Eudragit RS® in ratio of 2:1 gave the best modified release profile. Also, using di calcium phosphate as a binder and microcrystalline cellulose (Avicel PH 101) as a diluent resulted in a slower release profile comparing with other formulas. Based on the ʄ2 similarity factor value comparing with reference standard curve, F15, which contains Eudragit RL® and RS® in ratio of 2:1 with mannitol as a diluent and Avicel 102 as a binder, was selected as the best optimized formula. Drug release followed diffusion mechanism rather than erosion mechanism. Furthermore, FTIR spectra showed no possibility of chemical interaction between the drug and polymers, which were used in preparation of bilayer tablet. Stability studies of bilayer tablets under accelerated conditions indicated that the shelf life of the prepared tablets was 3 years and 5 months at 25 °C. However, stability of optimized formula in day light at room temperature revealed that the shelf life of oxcarbazepine is 1 year and 9 months.
Conclusion
The results of this study clearly demonstrate that oxcarbazepine can be successfully prepared as a bilayer modified release tablet.





















Similar content being viewed by others
References
Vyas SP, Khar RK. Controlled drug delivery concepts and advances. vallabh prakashan 2002; 1: 411–47.
Roni MA, Kibria G, Jalil R. Formulation and in vitro evaluation of alfuzosin extended-release tablet using directly compressible Eudragit. Ind J Pharm Sci. 2009;71(3):252–8.
Makhija SN, Vavia PR. Once daily sustained release tablets of venalafaxine, a novel antidepressant. Eur J Pharm Biopharm. 2002;54:9–15.
Kamble S, Poul B, Udapurkar P. Bilayer tablet of tramadol and gabapentine for combination pharmacotherapy of neuropathic pain: development and characterization. Int J App Pharm. 2018;10(3):100–7.
Sheetal M, Supriya S, Kadam V. Formulation, and evaluation of oxcarbazepine fast dissolve tablets. 2007;69(2):211–4.
Lloyd P, Flesch G, Dieterle. Clinical pharmacology and pharmacokinetics of oxcarbazepine. Epilepsia 1994;35(suppl 3): S10-S13.
Michael JM. Oxcarbazepine: mechanisms of action. In: Rene HL, Brain SM, Richard HM, Emilio P, editors. Antiepileptic Drugs. 5th ed. USA: Lippincott williams & wilking; 2002. p. 451–8.
Anupama K, Shelly K, Neena B. Formulation, and evaluation of mouth dissolving tablets of oxycarbazepine. Int J of Pharmacy and Pharma Sci. 2009;1(suppl 1):12–23.
Nirva VP, Narendra PC, Mayur PP. Formulation design of oxcarbazepine fast release tablets prepared by melt granulation technique. Asian J of Pharm 2008;2: 22–26.
Higuchi T, Connors KA. Phase solubility techniques. Advanced Analytical Chemistry of Instrumentation. 1965;4:117–212.
Rawlins EA. Bentleys textbook of pharmaceutics. 8th wd. U.K.: Bailliere Tindall 2006;641–649.
Biritish Pharmacopeia (BP). Electronic edition. London: Crown, Inc. 2007.
Odeku OA. Tablet Evaluation Tests.Pharmadepia 2008.
Paulo E, Renata O, Gerson P. Oxcarbazepine: validation and application of an analytical method Braz J Pharm Sci 2010; 46(2): 256–71.
Nirav P, Narendra C, Jayvadan P, Tejal S, Julan D, Rajnikant P. Comparison of in vitro dissolution profiles of oxcarbazepine-HP B-CD tablets formulations with marketed oxcarbazepine tablets. Dissolut Technol. 2008;15:28–34.
Naringreker GV, Arnold KA, Erkaboni D. Controlled-release formulations, method of manufacture, and use thereof, United States Patent 12/330280,2008.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanism of solute release from porous hydrophilic polymer. Int J Pharm. 1983;15:25–35.
Atul K, Ashok KT, Narendra KJ, Subheet J. Formulation, and in vitro evaluation of extended-release matrix tablets of zidovudine: influence of combination of hydrophilic and hydrophobic matrix formers. AAPS Pharm SciTech 2006; 7(1) Article 1: E1-E9.
Nahla SB, Ibrahim ME, Alanood SA. Controlled-release carbamazepine matrix granules and tablets compressing lipophilic and hydrophilic components. Drug Deliv. 2009;16(1):57–65.
Al-Taani BM, Tashtoush BM. Effect of microenvironment pH of swellable and erodible buffered matrices on the release characteristics of diclofenac sodium. AAPS Pharm Sci Tech. 2003;4:E43.
Mohan A, Sangeetha G. In vitro-in vivo evaluation of fast-dissolving tablets containing solid dispersion of oxcarbazepine. Int J Pharm Pharm Sci. 2016;8(8):124–31.
United States Pharmacopeia 29 (2005), <905> Uniformity of Dosage Units.
Fernando GT, Glenn A, Meyer MA, Ricci MA, Coppari A C, Gustavo A, Triple combination release multi-layered tablet, United States Patent 244473,2005.
Zhang Y, Law Y, Chakrabarti S. Physical properties and compact analysis of commonly used direct compression binders. AAPS Pharma Sci Tech 2003;4(4) Article 62.
Okor RS, Uhumwangho MU, Adogah JT. Potential of carnauba wax in ameliorating brittle fracture during tableting. Pack J Pharm Sci. 2009;22(1):58–61.
Basak SC, Kumar KS, Ramalingam M. Design, and release characteristics of sustained release tablet containing metformin HCl. Brazilian J Pharm Sci. 2008;44(3):477–83.
Saber M, Magnard F, Bouzon J, Vergnaud JM. Modeling of matter transfers in drug-polymer device used as galenic form. J Polym Eng. 1988;8:295–314.
Stamm A, Tritsch JC. Some consideration on the liberation of drugs from inert matrices. Drug Dev Ind Pharm. 1989;12:2337–53.
Talukder M, Shafiqur R, Tasnuva H. Statistical evaluation of hydrophobic polymer-based diclofenac sodium matrix tablet. Der Pharma Chemica. 2009;1(1):225–37.
Guyot M, Fawaz F. Nifedipine loaded polymeric microsphere: preparation and physical characteristics. Int J Pharm. 1998;175:61–74.
Kibbe AH. Handbook of Pharmaceutical excipients. Washington, DC, USA: American Pharmaceutical Association 2000.
Boza A, Caraballo I, Alvarez-Fuentes J, Rabasco AM. Evaluation of Eudragit RSPO and Ethocel 100 matrices for the controlled release of lobenzarit disodium. Drug Dev Ind Pharm. 1999;25(2):229–33.
Lee PI. Diffusional release of a solute from polymeric matrix-approximate analytical solutions. J Memb Sci. 1980;7:255–75.
Indranil KY, Hari PS, Rana PS, Dinesh C, Durga J, Jain DA, et al. Formulation and optimization of aceclofenac sustained release matrix tablets. Int J Pharm Tech. 2010;2(1):592–8.
Emami J, Tajeddin, Ahmadi F. Preparation and in vitro evaluation of sustained release matrix tablets of flutamide using synthetic and naturally occurring polymers. Iranian J Pharma Res 2008;7(4):247–256.
Swain RP, Kumari TR, Panda S. Formulation development and evaluation of sustained release ibuprofen tablets with acrylic polymers (Eudragit) and HPMC. Int J Pharm Pharm Sci; 8(2):131–5.
Katikaneni PR, Upadrashta SM, Neau SH, Mitra AK. Ethylcellulose matrix-controlled release tablets of water-soluble drug. Int J Pharm. 1995;123(1):119–25.
Hazneder S, Dortunc B. Preparation, and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int J Pharm. 2004;269(1):131–40.
Apu AS, Pathan AH, Shrestha D, Kibria G, Jalil R. Investigation of in vitro release kinetics of carbamazepine from Eudragit® RSPO and RLPO matrix tablets. Tropical J Pharm Res. 2009;8(2):145–52.
Sasidhar RLC, Vidyadhara S, Ramesh J, Nagaraju R, Prakash K. Formulation and evaluation of controlled release of losartan potassium matrix tablets using poly (ethyleneoxides). Curr Trends Biotechnol Pharm 2009;3(4): 440–446.
Skinner GW, Harcum WW, Durig T. Ethylcellulose in direct compression modified release tablets: impact of polymer structure and formulation variables. HERCULES: Aqualon Davison 2002;PTR-023.
Sinah VR, Agrawal MK, Kumria R. Influence of formulation and excipient variables on the pellet properties prepared by extrusion spheronization. Curr Drug Deliv. 2005;2(1):1–8.
Bastos O, Friedrich B, Beck R. Effects of filler-binders and lubricants on physicochemical properties of tablets obtained by direct compression: A22 factorial design. Lat Am J Pharm. 2008;27(4):578–83.
Rahman SMH, Telny TC, Ravi TK, Kuppusamy S. Role of surfactant, and pH in dissolution of curcumin. Ind J Pharm Sci. 2009;71(2):139–42.
Huang Y, Wu P, Chang J, Tsai M, Tsai Y. Design, and evaluation of sustained release microsphere of potassium chloride prepared by Eudragit. Eur J Pharm Sci. 2003;19(2–3):115–22.
Pamudji JS, Sjuib F, Ibrahim S, Sumirtapura Y. Study on release of dextromethorphan hydrobromide from tablet matrix of Eudragit RSPO into media of different pH. Acta Pharm Indon. 2005;30(3):89–93.
Moore J, Flanner H. Mathematical comparison of dissolution profiles. Pharm Tech. 1996;20(6):64–74.
Apurba SA, Atiqul HP, Golam K, Rezaul J. In vitro release kinetic study of theophylline from Eudragit RSPO and Eudragit RLPO matrix tablets. J Pharm Sci. 2009;8(1):1–6.
Silversteine R, Webster F, Kiemel D. Infrared spectrometry in spectrometric identification of organic compounds. 7th ed. John Wily and Sons: Inc; 2005. p. 72–126.
Patrick S. Chemical kinetics and stability in Martin’s physical pharmacy and pharmaceutical sciences. 5th ed. USA: Lippincott Williams and Wilkins; 2006. p:411.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ameen, M.S.M., Ibrahim, N.J. & Omar, T.A. Design and Evaluation of Sustained Release Bilayer Tablets of Oxcarbazepine. J Pharm Innov 18, 1213–1228 (2023). https://doi.org/10.1007/s12247-022-09694-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09694-2