Abstract
Gastroenteritis caused by Campylobacter represents the most common reported foodborne bacterial illness worldwide, followed by salmonellosis. Both diseases are often caused by the consumption of contaminated, insufficiently heated poultry meat. This can result from contamination of the meat during the slaughtering processes. Food contact surfaces like stainless steel or plucking fingers contribute significantly to cross-contamination of poultry carcasses. Modification of these surfaces could lead to a reduction of the bacterial burden, as already proven by successful application in various food industry sectors, such as packaging.In this study, nanoscale silica-coated and uncoated stainless-steel surfaces and plucking fingers were compared on a pilot scale regarding attachment and detachment of Campylobacter jejuni, Salmonella Enteritidis and Escherichia coli.The bacteria did not adhere less to the coated plucking fingers or stainless-steel sections than to the uncoated ones. The coating also did not lead to a significant difference in detachment of Campylobacter jejuni, Salmonella Enteritidis and Escherichia coli from the investigated surfaces compared to the uncoated ones.Our study did not reveal any differences between the coated and uncoated surfaces with regard to the investigated bacteria. In order to achieve a better adaptation of the coating to slaughterhouse conditions, future studies should focus on its further development based on the investigation of specific coating parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foodborne diseases are a global public health issue for human life and health. According to World Health Organization (2021), 600 million cases of illness are caused each year worldwide by eating contaminated food. In this context, diarrheal diseases are the most prevalent consequences. Campylobacter are considered the most frequent bacterial cause of gastroenteritis in humans worldwide, accounting for approximately 95 million cases per year, with Campylobacter jejuni (C. jejuni) being the most widespread human pathogenic species (Centers for Disease Control and Prevention 2023; World Health Organization 2015). Salmonellosis represents the second most common bacterial intestinal disease, whereby Salmonella enterica serovar Enteritidis (S. E.) is one of the two most relevant serotypes for humans (World Health Organization 2020). One of the major vehicles of Campylobacter and Salmonella infections in humans is contaminated poultry meat, which is consumed insufficiently heated (Robert Koch-Institut 2020). Efforts were made to prevent the occurrence of the bacteria, especially in primary production, through European-wide control programmes. Although Salmonella illnesses have declined, there are still high numbers of infections, and in the case of Campylobacter, the disease rate has stagnated at a constantly high level for years (European Food Safety Authority 2021 and European Centre for Disease Prevention and Control 2022; Statista 2022).
Slaughtering as the subsequent downstream part of the meat-producing process is a further focus for reducing bacterial contamination of poultry meat. Cross-contamination during slaughtering poses a particular risk, as potentially pathogenic material can be transferred from one carcass to the next via contact surfaces such as plucking fingers, guide elements or conveyor belts (Veluz et al. 2012). The most critical points in this context are plucking and evisceration, as these highly mechanised processes can lead to an increased exchange of organic material such as feathers, excreta and intestinal content between machines and carcasses (Gruntar et al. 2015; Rasschaert et al. 2007). Either by the pressure of the plucking fingers on carcasses or by the mechanical action of the evisceration equipment, faeces can leak out and be spilled over equipment surfaces to contaminate subsequent carcasses (Pacholewicz et al. 2015; Zeng et al. 2021). Animal carcasses’ contact surfaces of the slaughterhouse are, therefore, of particular importance with regard to possible cross-contamination and transmission of pathogens (Arnold and Silvers 2000). It is well known that pathogenic bacteria such as Salmonella and Campylobacter are able to colonise surfaces commonly used in food industry, such as stainless steel, rubber or glass (Chia et al. 2009). Modifying these surfaces to prevent the adhesion of bacteria and facilitate their detachment could contribute to an overall reduction of the bacterial load in the slaughter process. Certain natural surfaces are able to reduce bacterial attachment, mostly due to an ultra-thin nanoscale topography, which is why the development of such a surface is also a promising approach in industry (Ivanova et al. 2012). Initial applications on exposed surfaces based on nanotechnology are already well-established practice both in the medical sector and on packaging in the food industry (Chouirfa et al. 2019; Gallocchio et al. 2016). The most promising way to achieve nanostructuring of slaughterhouse surfaces appears to be the application of a coating, as this can be very specifically adapted to the desired effects and respective conditions with regard to the material composition and the modification of the surface structure. A variety of materials, including synthetic materials such as polyurethane (Bakker et al. 2004) and metals like gold (Díaz et al. 2007), have already been tested for their suitability as coating materials. Another material that is being considered for use in this area is silicon dioxide. In addition to already known advantages such as good biocompatibility and low manufacturing costs, a certain effectiveness against bacteria has also been demonstrated in various studies (El-Shetehy et al. 2021; Rao et al. 2005).
In previous studies, small, tending and partly significant effects of nanoscale silicon dioxide coating were observed for S. E., E. coli, Listeria monocytogenes and C. jejuni in a laboratory scale (Blaeske et al. 2023; Hillig et al. 2024; Schumann-Muck et al. 2023a, b). We hypothesised that these effects will also be present in a more practical approach in a pilot plant scale. The aim of this study was, therefore, to investigate the effects of this nanoscale coating on stainless-steel slaughterhouse equipment and plucking fingers on the attachment and detachment of S. E., C. jejuni and E. coli on a pilot plant in a more practical environment. E. coli was included in the investigations as an indicator bacterium, as its presence on slaughterhouse surfaces can indicate possible faecal contamination and thus also with Salmonella or Campylobacter (Foley et al. 2008).
Materials and methods
Bacterial material
S. E. isolate 19-SA00115, E. coli isolate 20-AB00467 and C. jejuni isolate BFR-CA-19285 were obtained from the German Federal Institute for Risk Assessment (BfR, Berlin, Germany) and originated from chicken meat samples. They were cryopreserved at the Institute of Food Hygiene Leipzig at -80 °C (Cryobank, Mast Group Ltd., Germany). Fresh working cultures of S. E. and E. coli were obtained as described elsewhere (Schumann-Muck et al. 2023b) and used for a fortnight. Overnight cultures were grown to a cell density of about 108 cfu per mL as described previously (Schumann-Muck et al. 2023b) except for the addition of bovine serum albumin. C. jejuni working cultures were obtained by spreading cryopreserved beads on blood agar plates (Columbia Agar with Sheep Blood Plus; Oxoid GmbH, Germany) for each experimental approach and incubating them microaerobically (85% N2, 10% CO2, 5% O2) for 36 h at 42 °C. Therefore, the TRILAB system (TRILAB; Jenny Science AG, Switzerland) was used. The bacterial colonies from these plates were then stirred into Brain Heart Infusion Broth (BHI Broth; TN1216; sifin diagnostics GmbH, Germany) and incubated without agitation microaerobically for 16 h at 42 °C to a cell density of about 108 cfu per mL.
On-site at the pilot scale slaughtering unit, the suspensions of S. E., E. coli and C. jejuni were combined in a 1:1:1 ratio for every experimental approach to obtain a pool of target organisms.
Surfaces
The experiments were carried out in the technical centre of the German Federal Institute for Risk Assessment (BfR, Berlin, Germany). Commercial plucking fingers made of thermoplastic rubber with the following data were used: 20 mm bore diameter; hard version; total length, 97.5 mm; (Westfalia Werkzeug company GmbH & Co. KG, Germany). Before being used in the experiments, the plucking fingers were pretreated by cleaning and sterilisation. For this purpose, they were first placed in 5% Decon solution (Decon™ Decon90, Fisher Scientific GmbH, Germany), then rinsed with distilled water and finally degreased in a 95% 2-propanol solution (Carl Roth GmbH, Germany). After a further rinse with distilled water, they were first dried in a biosafety cabinet and then autoclaved. A more detailed description of this treatment can be found at Schumann-Muck et al. (2023a). In order to produce the coated plucking fingers used in this study, a nanoscale layer of silicon dioxide was applied by Nanopool GmbH (Germany) utilising their commercial product Liquid Glass Metall. Afterwards, the same number of coated and uncoated fingers were inserted into a drum plucker (plucking drum, 92 cm diameter, 42 cm height, equipped with 258 plucking fingers).
Two stainless-steel rods (type 304; X5CrNi1810) with a diameter of 30 mm and a length of 110 cm were custom-manufactured by SKS (Sondermaschinen- und Fördertechnikvertriebs-GmbH, Germany). They were divided into sections of 10 cm in length, and these sections were then alternately left uncoated or coated with a nanoscale layer of silicon dioxide applied by Nanopool GmbH, Germany, using its commercial product Liquid Glass Metall.
One rod was then fixed at a mobile carcass rack for attachment experiments in such a way that it was at a height of 140 cm parallel to the ground. For the detachment experiments, the second rod was attached parallel above the first one at a distance of 6 cm, but offset by one section, so that coated and uncoated sections were directly above each other.
Study design and recovery of the bacteria
The broilers used for the experiments were purchased already killed from a commercial poultry processor and used for experiments within a maximum of 2 h after killing.
Attachment and detachment tests with plucking fingers were repeated four times each using five uncoated and five coated fingers. Five broilers, taken from slaughter line after stunning and bleeding, were used for every finger experiment. These were scalded for 3 min in tap water at about 53 °C (Brühanlage Typ L1000, Westerhoff Geflügeltechnik GmbH, Germany). After that, each broiler was inoculated using a pipette with 10 mL mixed bacterial suspension, which was distributed over the entire carcass. Then, these five broilers were defeathered together for 30 to 40 s in the drum plucker equipped with coated and uncoated plucking fingers. Afterwards, the carcasses were removed and discarded, and the respective ten plucking fingers to be examined were taken from the machine. The detachment experiments followed subsequently. Therefore, the already used and, thus, contaminated plucking machine was first given a coarse cleaning with tap water at 15 °C for 15 s using a hand shower to soak and loose coarse organic soiling such as feathers. This was followed by the actual cleaning in form of high-pressure cleaning at 130 bar and a water temperature of 75 °C for 1 min (HDS 9/18–4 M, Alfred Kärcher Vertriebs-GmbH, Germany). Recovering bacteria from plucking fingers for attachment and detachment experiments was carried out following the method of Arnold. The tips of the fingers were cut off behind the third rib with sterile scissors, placed in a 50-mL centrifuge tube (centrifuge tube 50, TPP, Switzerland) filled with 10 mL sodium chloride peptone solution and manually shaken for 15 to 20 s. They were kept refrigerated at 5 °C for a maximum of 6 h until further processing. Then decimal dilutions of the liquids were spread on the appropriate selective nutrient media for detection of each of the three bacteria used and incubated for 24 h at the optimal growth temperatures respectively: E. coli on Tryptone Bile X Glucuronic Agar (TBX; TN1259; sifin diagnostics GmbH, Germany) at 42 °C, S. E. on Xylose Lysine Deoxycholate Agar (XLD; 85,864.5000; VWR International GmbH, Germany) at 37 °C and C. jejuni on modified Cefaperazone Charcoal Desoxycholate Agar plates (mCCDA, Oxoid GmbH, Germany) microaerobically at 42 °C.
Experiments for attachment to the stainless-steel rod were repeated four times and performed with three broiler carcasses each, which were taken from a slaughter line after plucking. These were inoculated in the breast area using a pipette with 3.0 mL of the bacterial suspension, which was distributed with sterile spatulas. Carcasses were then hooked into a slaughter belt, which moved at a speed of 0.1 m/s. This caused them to move along the stainless-steel rod, thereby touching it at the inoculated breast area. Then, six consecutive sections, and thus alternately coated and uncoated sections, were sampled on the rod. For detachment experiments, a broiler was inoculated in the breast area with 3.0 mL of the bacterial suspension and moved along the rods with the aid of the slaughter belt in such a way that both rods were touched simultaneously with the inoculated breast area. Afterwards, the broiler was inoculated a second time and passed along the rods again. The two rods attached one above the other were then rinsed with 1.5 l water (76–77 °C). For each test, the first touched section of each rod was sampled, and thus a coated and an uncoated section were sampled at the same time. This was repeated 10 times.
According to DIN EN ISO 18593, bacteria were recovered from the rod by sampling each section vertically and horizontally for 30 s by wi** with uniformly strong pressure using a sterile touch sponge (Polywipe with Peptone Saline; Medical Wire and Equipment, UK). The sponge was then placed in a plastic bag filled with 90 mL sodium chloride peptone solution, which was sealed to prevent leakage and kept refrigerated at 5° C for a maximum of 6 h until further processing. Afterwards, the bags were stomached for 1 min at 260 rpm (Stomacher 400 Circulator, Seward Ltd., UK). Decimal dilutions were then prepared from the liquids, which were plated and incubated on the appropriate selective culture media for the bacteria used, as described for the plucking fingers.
Statistics
Bacterial counts were log10 transformed to perform the statistical analysis. Attachment and detachment of S. E., E. coli and C. jejuni from plucking fingers was calculated as the average of four replicates each from the recovered bacterial counts of five fingers per replicate. The attachment and detachment values represent the number of bacteria that were able to attach to the fingers or could not detach from them by the rinsing process. Differences between coated and uncoated plucking fingers per treatment were statistically analysed with an unpaired t-test at an alpha level of significance of 0.05.
For attachment of the target organisms to the stainless-steel rod, the section average bacterial counts of four replicates were used to calculate linear regression of fit. Linear regression lines of coated and uncoated steel were compared based on slope and intersect following the procedure described by Glantz (2012) to generate F values for reference at an alpha level of significance of 0.05.
Detachment of the bacteria from the stainless-steel rod was calculated as the average of 10 replicates from the recovered bacteria counts of one section per replicate. The detachment values represent the number of bacteria that were able to remain attached to the rod after rinsing. Differences between coated and uncoated rod sections per treatment were statistically analysed using an unpaired t-test at an alpha level of significance of 0.05.
Except comparison of linear regression lines, all statistical analyses were carried out using Prism 9 (GraphPad Software, LLC, USA).
Results
Plucking fingers
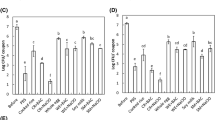
Attachment tests on the plucking fingers resulted in S. E. attachment of 3.724 log10 cfu on uncoated and 3.691 log10 cfu on coated fingers. This difference was not statistically significant (p = 0.896; Fig. 1). Also, no statistically significant difference (p = 0.165) was detected between uncoated and coated plucking fingers for E. coli (3.863 log10 cfu vs. 3.649 log10 cfu; Fig. 1). The same results were observed for C. jejuni (3.067 log10 cfu vs. 2.938 log10 cfu; p = 0.564; Fig. 1).
After rinsing, similar numbers of S. E. were still adhering to the uncoated and coated plucking fingers (2.327 log10 cfu vs. 2.401 log10 cfu, respectively, p = 0.517; Fig. 2). The same effect was observed for E. coli (2.391 log10 cfu vs. 2.256 log10 cfu, p = 0.105; Fig. 2) and C. jejuni (2.408 log10 cfu vs. 2.302 log10 cfu, p = 0.479; Fig. 2).
Stainless steel
The mean values for attachment of the respective bacteria on the sections examined on the rod can be taken from Table 1. Due to a smearing effect caused by the carcasses sliding along the rod, linear regression lines were determined from the mean values for the uncoated and coated sections, which were used for the statistical evaluation. Assuming differences in attachment regarding the coating, the regression lines based on bacterial counts on coated or uncoated stainless-steel sections would differ significantly in slope and/or intercept. The procedure mentioned in the data analysis section compares regression lines in regard to these two characteristics, yielding in an F value. At an alpha level of significance of 0.05, for νn = 2 and νd = 2, the resulting F value needs to exceed 19.00. The regression line for S. E for the coated sections was y = − 0.2671x + 7.2239 and for the uncoated sections y = − 0.3371x + 7.3641. This resulted in an F value of 0.987. For E. coli, the equation y = − 0.2850x + 7.0100 for the uncoated sections and y = − 0.2152x + 6.8285 for the coated ones resulted in an F value of 1.290 and for C. jejuni, the regression line for the coated sections was y = − 0.4557x + 6.7429 and for the uncoated sections y = − 0.4562x + 6.7243 (F value = 0.003). In no case, the F value exceeded 19.00, so there were no statistical differences in attachment for any bacteria in regard of coating.
Regarding detachment, there was a slight difference in S. E. between the non-detached bacteria of the uncoated (3.423 log10 cfu) and coated (3.002 log10 cfu) sections, but this was not found to be statistically significant (p = 0.205; Fig. 3). In the case of E. coli, no statistically significant difference was found between coated and uncoated sections in the still attached bacteria either (2.840 log10 cfu vs. 2.937 log10 cfu; p = 0.82; Fig. 3). For C. jejuni, 2.768 log10 cfu on the uncoated and 2.646 log10 cfu on the coated stainless-steel sections were unable to detach. However, this did not represent a statistically significant difference (p = 0.387; Fig. 3).
Discussion
Plucking fingers
In this study, silicon dioxide coating of plucking fingers did not result in less attachment or better detachment for S. E., E. coli and C. jejuni. Similar results were already reported by Schumann-Muck et al. (2023a) and Blaeske et al. (2023) after examining the same coating on plucking fingers on a laboratory scale. All of these findings are plausible considering the results of Arnold and Silvers (2000), who reported that plucking fingers, due to the rubber used for their manufacture, showed lower bacterial adhesion and, thus, less biofilm formation compared to other materials, e.g. stainless steel. The applied silica coating of the plucking fingers, therefore, does not improve the material properties with regard to the attachment of the bacteria due to the covering of the already bacteria-reducing material.
Despite the already existing laboratory results, the tests were carried out on a pilot plant scale, as a possible influence of the test conditions in the technical centre, such as the use of a plucking machine, could not be fundamentally ruled out. This is particularly important with regard to the use of a plucking machine. One reason why the plucking step is mentioned in several sources as one of the main causes of cross-contamination, despite the bacteria-reducing properties of the plucking finger rubber, may be airborne spread of bacteria via aerosols or water droplets. One source for this could be the water flushing during plucking process, which leads to aerosol formation and, thus, to a greater spread of bacterial contamination even outside of the plucking machine, as Allen et al. (2003) confirmed in their study. They also observed that the bacterial distribution pattern was affected by the motion of the plucking fingers and the design of the plucking machine, because more E. coli were found on carcasses after plucking with a contra-rotating machine (mean log10 5.23 ± 0.09 cfu/mL carcass rinse for single pass) than with a disc machine (mean log10 2.43 ± 0.41 cfu/mL carcass rinse), which made the former less effective in removing the marker organism. These considerations must also be taken into account in our study, as a drum plucker operating on the principle of a disc machine was used for our experiments. Due to its construction, it is possible that the contact of the five simultaneously plucked broilers with each other and with the plucking fingers during the 40 s lasting plucking process led to increased contamination of the fingers. The in-process water in combination with the rapid rotation and the closed lid of the machine also led to a strong distribution of water within the plucker and to aerosol formation, which may also have influenced the distribution of bacteria. These points must be taken into account when assessing the test results, as the use of the coated fingers in a plucking belt, which is most commonly used in commercial slaughterhouses, would possibly produce different results compared to the use in the drum plucker.
Stainless steel
In the present study, nanoscale coating did not significantly reduce bacterial adhesion to stainless-steel rod section. This result is in contrast with the study of Puckett et al. (2010) who found that nanorough titanium surfaces had a statistically significant reduction in attachment of all bacteria they examined compared to conventional titanium (p < 0.01). Thus, it is reasonable to reduce bacterial attachment to stressed surfaces by means of modifications. This is also particularly important for stainless steel as was examined in the present study. Although stainless steel is frequently used in the food industry due to its relatively low initial costs and high corrosion resistance (Schmidt et al. 2012), Flint et al. (2000) were able to show that, compared to other surfaces such as glass, stainless steel provides a good substrate for bacteria such as Streptococcus thermophilus to adhere to. For this reason, attempts have already been made with different types of stainless-steel surface modification. A nanoscale amorphous SiOxCyHz coating proved to be successful, as Di Cerbo et al. (2021) were able to demonstrate a 5-log decrease of E. coli and S. Typhimurium compared to the uncoated surfaces.
One reason for the difference between the results of those studies and the current one could be the contact time of the bacteria with stainless steel. Since the conditions in the present study intended to mimic those in the slaughterhouse, the contact time of inoculated carcasses while sliding along the rod was only a few seconds. In other studies, exposure times ranging from 30 min (Zakarienė et al. 2018) to 1 h (Schlisselberg and Yaron 2013) were chosen for initial bacterial attachment to the surfaces, resulting in reduced attachment on the treated surfaces.
When investigating bacterial detachment from the stainless-steel rod using hot water, no improvement was demonstrated by the coating in the present study. These results are in contrast with the report by Ivanova et al. (2012) who were able to show that cicada wings with a naturally ultra-thin nanostructured topography offer good protection against Pseudomonas aeruginosa, as they exhibited a water-repellent activity and thus a kind of self-cleaning effect. The development of surface-modifying coatings based on these findings is often related to the modification of relevant surface properties such as surface free energy, roughness and especially surface wettability. One measure of this is the water contact angle, which, as shown by Schumann-Muck et al. (2023b), increases from 56.7 to 115.4° when the identical silicon dioxide coating was applied to stainless-steel discs. This results in a hydrophobic character of the surface with a lower wettability, which should actually lead to an increased detachment of the bacteria from the coated surface. One reason that the results of the present study are different could be due to the organic material transferred to the rod by the carcasses, whose most important components in this context are fat and proteins. High protein concentrations are able to influence the interaction of bacteria and surfaces (Singh et al. 2011). On the one hand, the proteins can act as a passivating layer and thus lead to a reduction in bacterial adhesion to the nanoscale surface. On the other hand, a flattening effect was observed due to the protein layer formed, which could lead to a reduction in the function of the nanoscale coating. This flattening effect could confirm our results, as the proteins of the chicken carcasses could reduce the hydrophobic effect of the applied nanoscale coating. As a result, the detachment of the bacteria is made more difficult. The results generated in the present study are consistent with previously published results investigating the detachment of various bacteria from stainless steel coated with nanoscale silicon dioxide (Blaeske et al. 2023; Hillig et al. 2024; Schumann-Muck et al. 2023b).
In comparison to previous laboratory-scale experiments, surfaces were contaminated by direct carcass contact. Thus, the coating would rather affect the contamination of organic material (including the bacteria) than the bacteria itself when applied as a suspension. The area of organic contamination was not always recognisable on stainless-steel rods and not at all on plucking fingers. Furthermore, a greater area than only the contacting area was sampled. Here, also bacterial suspension could have been included, which was eventually spilled onto the surface due to the carcass motion (especially in the plucking machine). Therefore, the results would represent a combined effect on solely bacterial adhesion/detachment and on organic material contamination and cleaning.
It must be noted that it is questionable whether the results of this study can be generalised for the bacteria used, as only one strain of each bacterium was used. Especially in the case of Campylobacter, it is known that there are differences between strains, for example with regard to colonisation ability and survivability (Park 2002). For this reason, the results presented here are difficult to transfer to other strains.
Conclusion
In the current study, the effects of a nanoscale silica coating on the attachment and detachment of S. E., E. coli and C. jejuni were investigated on a pilot scale by comparing coated and uncoated plucking fingers and stainless-steel surfaces.
The coating did not lead to reduced attachment or better detachment of the bacteria strains examined regardless of the examined surface material.
In order to reduce the bacterial contamination of chicken meat, the modification of slaughterhouse surfaces particularly affected by cross-contamination with the aid of coatings could represent a possible approach. However, as can be seen from the available results, the silicon dioxide coating investigated here cannot be recommended for use at the present time for the purpose of reducing S. E., E. coli and C. jejuni in slaughterhouses. Since in general, a bacteria-reducing effect has been described for several nanoscale coatings, several parameters of such coatings should be focussed in future research to achieve a potential effect for the respective bacteria in the poultry slaughterhouse environment. Such parameters could include the coating material as well as the structure and formation of the nanolayer, or the resulting roughness. Analyses of durability of the coating could also be useful from an application perspective.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Allen VM, Tinker DB, Hinton MH, Wathes CM (2003) Dispersal of micro-organisms in commercial defeathering systems. Br Poult Sci 44:53–59. https://doi.org/10.1080/0007166031000085436
Arnold JW, Silvers S (2000) Comparison of poultry processing equipment surfaces for susceptibility to bacterial attachment and biofilm formation. Poult Sci 79:1215–1221. https://doi.org/10.1093/ps/79.8.1215
Bakker DP, Busscher HJ, van Zanten J, de Vries J, Klijnstra JW, van der Mei HC (2004) Multiple linear regression analysis of bacterial deposition to polyurethane coatings after conditioning film formation in the marine environment. Microbiology 150:1779–1784. https://doi.org/10.1099/mic.0.26983-0
Blaeske V, Schumann-Muck FM, Hamedy A, Braun PG, Koethe M (2023) Campylobacter colonisation of slaughterhouse surfaces may be affected by ultra-thin silica coating. AIMSAGRI 9:52–68. https://doi.org/10.3934/agrfood.2024004
Centers for Disease Control and Prevention (2023) Information for Health Professionals | Campylobacter | CDC
Chia TWR, Goulter RM, McMeekin T, Dykes GA, Fegan N (2009) Attachment of different Salmonella serovars to materials commonly used in a poultry processing plant. Food Microbiol 26:853–859. https://doi.org/10.1016/j.fm.2009.05.012
Chouirfa H, Bouloussa H, Migonney V, Falentin-Daudré C (2019) Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater 83:37–54. https://doi.org/10.1016/j.actbio.2018.10.036
Di Cerbo A, Mescola A, Rosace G, Stocchi R, Rossi G, Alessandrini A, Preziuso S, Scarano A, Rea S, Loschi AR, Sabia C (2021) Antibacterial effect of stainless steel surfaces treated with a nanotechnological coating approved for food contact. Microorganisms. https://doi.org/10.3390/microorganisms9020248
Díaz C, Schilardi PL, Salvarezza RC, Lorenzo F, de Mele M (2007) Nano/microscale order affects the early stages of biofilm formation on metal surfaces. Langmuir 23:11206–11210. https://doi.org/10.1021/la700650q
El-Shetehy M, Moradi A, Maceroni M, Reinhardt D, Petri-Fink A, Rothen-Rutishauser B, Mauch F, Schwab F (2021) Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat Nanotechnol 16:344–353. https://doi.org/10.1038/s41565-020-00812-0
European Food Safety Authority 2021, European Centre for Disease Prevention and Control (2022) The European Union One Health. Zoonoses Report 20:1–273. https://doi.org/10.2903/j.efsa.2022.7666
Flint SH, Brooks JD, Bremer PJ (2000) Properties of the stainless steel substrate, influencing the adhesion of thermo-resistant streptococci. J Food Eng 43:235–242. https://doi.org/10.1016/S0260-8774(99)00157-0
Foley SL, Lynne AM, Nayak R (2008) Salmonella challenges: prevalence in swine and poultry and potential pathogenicity of such isolates. J Anim Sci 86:E149–E162. https://doi.org/10.2527/jas.2007-0464
Gallocchio F, Cibin V, Biancotto G, Roccato A, Muzzolon O, Carmen L, Simone B, Manodori L, Fabrizi A, Patuzzi I, Ricci A (2016) Testing nano-silver food packaging to evaluate silver migration and food spoilage bacteria on chicken meat. FOOD ADDIT CONTAM A 33:1063–1071. https://doi.org/10.1080/19440049.2016.1179794
Glantz SA (2012) Overall test for coincidence of two regression lines. In: Primer of biostatistics, 7th edn. McGraw-Hill, New York, p 161
Gruntar I, Biasizzo M, Kušar D, Pate M, Ocepek M (2015) Campylobacter jejuni contamination of broiler carcasses: population dynamics and genetic profiles at slaughterhouse level. Food Microbiol 50:97–101. https://doi.org/10.1016/j.fm.2015.03.007
Hillig N, Schumann-Muck F, Hamedy A, Braun PG, Koethe M (2024) Impact of nanoscale silicon dioxide coating of stainless-steel surfaces on Listeria monocytogenes. Folia Microbiol 69:173–180. https://doi.org/10.1007/s12223-023-01089-1
Ivanova EP, Hasan JK, Webb H, Truong VK, S. Watson GA, Watson J, Baulin VA, Pogodin S, Wang JY, Tobin MJ, Löbbe C, Crawford RJ (2012) Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 8:2489–2494. https://doi.org/10.1002/smLl.201200528
Pacholewicz E, Swart A, Schipper M, Gortemaker BGM, Wagenaar JA, Havelaar AH, Lipman LJA (2015) A comparison of fluctuations of Campylobacter and Escherichia coli concentrations on broiler chicken carcasses during processing in two slaughterhouses. Int J Food Microbiol 205:119–127. https://doi.org/10.1016/j.ijfoodmicro.2015.04.006
Park SF (2002) The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int J Food Microbiol 74:177–188. https://doi.org/10.1016/S0168-1605(01)00678-X
Puckett SD, Taylor E, Raimondo T, Webster TJ (2010) The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomater Sci 31:706–713. https://doi.org/10.1016/j.biomaterials.2009.09.081
Rao KS, El-Hami K, Kodaki T, Matsushige K, Makino K (2005) A novel method for synthesis of silica nanoparticles. J Colloid Interface Sci 289:125–131. https://doi.org/10.1016/j.jcis.2005.02.019
Rasschaert G, Houf K, de Zutter L (2007) Impact of the slaughter line contamination on the presence of Salmonella on broiler carcasses. J Appl Microbiol 103:333–341. https://doi.org/10.1111/j.1365-2672.2006.03248.x
Robert Koch-Institut (RKI) (2020) Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2019, S. 1–255. https://doi.org/10.25646/6948
Statista (2022) Anzahl der jährlich registrierten Salmonellose-Erkrankungen in Deutschland in den Jahren 2001 bis 2019. Accessed 10 January 2022. https://de.statista.com/statistik/daten/studie/2673/umfrage/salmonellen-anzahl-von-erkrankungen-seit-2001/
Schlisselberg DB, Yaron S (2013) The effects of stainless steel finish on Salmonella Typhimurium attachment, biofilm formation and sensitivity to chlorine. Food Microbiol 35:65–72. https://doi.org/10.1016/j.fm.2013.02.005
Schmidt R, Erickson D, Sims S, Wolff P (2012) Characteristics of food contact surface materials: stainless steel. Food Prot Trends 32:574–584
Schumann-Muck FM, Blaeske V, Braun PG, Koethe M (2023a) Effectiveness of nanoscale silicon dioxide-coated picker fingers on Salmonella Enteritidis and Escherichia coli. Eur Food Res Technol. https://doi.org/10.1007/s00217-023-04378-8
Schumann-Muck FM, Hillig N, Braun PG, Griebel J, Koethe M (2023b) Impact of nanoscale coating of stainless steel on Salmonella Enteritidis and Escherichia coli. J Food Safety. https://doi.org/10.1111/jfs.13075
Singh AV, Vyas V, Patil R, Sharma V, Scopelliti PE, Bongiorno G, Podestà A, Lenardi C, Gade WN, Milani P (2011) Quantitative characterization of the influence of the nanoscale morphology of nanostructured surfaces on bacterial adhesion and biofilm formation. PLoS ONE 6:e25029. https://doi.org/10.1371/journal.pone.0025029
Veluz GA, Pitchiah S, Alvarado CZ (2012) Attachment of Salmonella serovars and Listeria monocytogenes to stainless steel and plastic conveyor belts. Poult Sci 91:2004–2010. https://doi.org/10.3382/ps.2011-01689
World Health Organization (2015) WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference grou. World Health Organization, Geneva, pp 2007–2015
World Health Organization (2020) Salmonella (non-typhoidal). Accessed 1 July 2020. https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal)
World Health Organization (2021) Estimating the burden of foodborne diseases. Accessed 6 January 2021. https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases
Zakarienė G, Novoslavskij A, Meškinis Š, Vasiliauskas A, Tamulevičienė A, Tamulevičius S, Alter T, Malakauskas M (2018) Diamond like carbon Ag nanocomposites as a control measure against Campylobacter jejuni and Listeria monocytogenes on food preparation surfaces. Diam Relat Mater 81:118–126. https://doi.org/10.1016/j.diamond.2017.12.007
Zeng H, de Reu K, Gabriël S, Mattheus W, de Zutter L, Rasschaert G (2021) Salmonella prevalence and persistence in industrialized poultry slaughterhouses. Poult Sci 100:100991. https://doi.org/10.1016/j.psj.2021.01.014
Acknowledgements
The authors would like to thank Felix Reich, Josephine Pech and Dirk Mayer from the German Federal Institute for Risk Assessment, Gunter Brendel and Christian Schmidt from SKS Sondermaschinen- und Fördertechnikvertriebs-GmbH and Romy Wohlfahrt from Märkische Geflügelhof-Spezialitäten GmbH.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled and organised by Projekt DEAL. The study was supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support programme (grant number: 281C104D18).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Victoria Bläske and Felicitas Schumann-Muck. The analyses were performed by Victoria Blaeske, Felicitas Schumann-Muck and Martin Koethe. The first draft of the manuscript was written by Victoria Blaeske, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human subjects.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blaeske, V., Schumann-Muck, F.M., Hamedy, A. et al. Influence of a nanoscale coating on plucking fingers and stainless steel on attachment and detachment of Salmonella Enteritidis, Escherichia coli and Campylobacter jejuni. Folia Microbiol (2024). https://doi.org/10.1007/s12223-024-01162-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12223-024-01162-3