Abstract
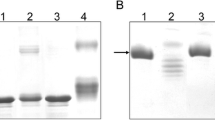
This study analyzed the interaction of commercial monoclonal anti-methylglyoxal antibodies that predominantly recognize argpyrimidine with unmodified and modified model proteins and small heat shock proteins. These antibodies specifically recognize methylglyoxal (MG)-modified bovine serum albumin and lysozyme, but they react equally well with both unmodified and MG-modified HspB1. Mutation R188W decreased the interaction of these antibodies with unmodified HspB1, thus indicating that this residue participates in the formation of antigenic determinant. However, these antibodies did not recognize either short (ESRAQ) or long (IPVTFESRAQLGGP) peptides with primary structure identical to that at Arg188 of HspB1. Neither of the peptides obtained after the cleavage of HspB1 at Met or Cys residues were recognized by anti-argpyrimidine antibodies. This means that unmodified HspB1 contains a discontinuous epitope that includes the sequence around Arg188 and that this epitope is recognized by anti-argpyrimidine antibodies in unmodified HspB1. Incubation of HspB1 with MG is accompanied by the accumulation of hydroimidazolones, but not argpyrimidines. Therefore, conclusions based on utilization of anti-argpyrimidine antibodies and indicating that HspB1 is the predominant and preferential target of MG modification in the cell require revision.







Similar content being viewed by others
References
Ahmed N, Dobler D, Dean M, Thornalley PJ (2005) Peptide map** identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem 280:5724–5732. https://doi.org/10.1074/jbc.M410973200
Antognelli C, Palumbo I, Aristei C, Talesa VN (2014) Glyoxalase I inhibition induces apoptosis in irradiated MCF-7 cells via a novel mechanism involving Hsp27, p53 and NF-kappaB. Br J Cancer 111:395–406. https://doi.org/10.1038/bjc.2014.280
Bair WB 3rd, Cabello CM, Uchida K, Bause AS, Wondrak GT (2010) GLO1 overexpression in human malignant melanoma. Melanoma Res 20:85–96. https://doi.org/10.1097/CMR.0b013e3283364903
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brock JW, Cotham WE, Thorpe SR, Baynes JW, Ames JM (2007) Detection and identification of arginine modifications on methylglyoxal-modified ribonuclease by mass spectrometric analysis. J Mass Spectrom 42:89–100. https://doi.org/10.1002/jms.1144
Chakraborty S, Karmakar K, Chakravortty D (2014) Cells producing their own nemesis: understanding methylglyoxal metabolism. IUBMB Life 66:667–678. https://doi.org/10.1002/iub.1324
Chalova AS, Sudnitsyna MV, Strelkov SV, Gusev NB (2014) Characterization of human small heat shock protein HspB1 that carries C-terminal domain mutations associated with hereditary motor neuron diseases. Biochim Biophys Acta 1844:2116–2126. https://doi.org/10.1016/j.bbapap.2014.09.005
Chen Z, Earl P, Americo J, Damon I, Smith SK, Zhou YH, Yu F, Sebrell A, Emerson S, Cohen G, Eisenberg RJ, Svitel J, Schuck P, Satterfield W, Moss B, Purcell R (2006) Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc Natl Acad Sci U S A 103:1882–1887. https://doi.org/10.1073/pnas.0510598103
Gao Y, Wang Y (2006) Site-selective modifications of arginine residues in human hemoglobin induced by methylglyoxal. Biochemistry 45:15654–15660. https://doi.org/10.1021/bi061410o
Gawlowski T, Stratmann B, Stork I, Engelbrecht B, Brodehl A, Niehaus K, Körfer R, Tschoepe D, Milting H (2009) Heat shock protein 27 modification is increased in the human diabetic failing heart. Horm Metab Res 41:594–599. https://doi.org/10.1055/s-0029-1216374
Gerasimovich ES, Strelkov SV, Gusev NB (2017) Some properties of three alphaB-crystallin mutants carrying point substitutions in the C-terminal domain and associated with congenital diseases. Biochimie 142:168–178. https://doi.org/10.1016/j.biochi.2017.09.008
Han J, Baik N, Kim KH, Yang JM, Han GW, Gong Y, Jardi M, Castellino FJ, Felez J, Parmer RJ, Miles LA (2011) Monoclonal antibodies detect receptor-induced binding sites in Glu-plasminogen. Blood 118:1653–1662. https://doi.org/10.1182/blood-2010-11-316943
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Muranova LK, Weeks SD, Strelkov SV, Gusev NB (2015) Characterization of mutants of human small heat shock protein HspB1 carrying replacements in the N-terminal domain and associated with hereditary motor neuron diseases. PLoS One 10:e0126248. https://doi.org/10.1371/journal.pone.0126248
Nefedova VV, Muranova LK, Sudnitsyna MV, Ryzhavskaya AS, Gusev NB (2015) Small heat shock proteins and distal hereditary neuropathies. Biochemistry 80:1734–1747. https://doi.org/10.1134/S000629791513009X
Oya T, Hattori N, Mizuno Y, Miyata S, Maeda S, Osawa T, Uchida K (1999) Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxal-arginine adducts. J Biol Chem 274:18492–18502
Oya-Ito T, Liu BF, Nagaraj RH (2006) Effect of methylglyoxal modification and phosphorylation on the chaperone and anti-apoptotic properties of heat shock protein 27. J Cell Biochem 99:279–291. https://doi.org/10.1002/jcb.20781
Oya-Ito T, Naito Y, Takagi T, Handa O, Matsui H, Yamada M, Shima K, Yoshikawa T (2011) Heat-shock protein 27 (Hsp27) as a target of methylglyoxal in gastrointestinal cancer. Biochim Biophys Acta 1812:769–781. https://doi.org/10.1016/j.bbadis.2011.03.017
Padival AK, Crabb JW, Nagaraj RH (2003) Methylglyoxal modifies heat shock protein 27 in glomerular mesangial cells. FEBS Lett 551:113–118
Rabbani N, Thornalley PJ (2012) Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids 42:1133–1142. https://doi.org/10.1007/s00726-010-0783-0
Rabbani N, Xue M, Thornalley PJ (2016) Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj J 33:513–525. https://doi.org/10.1007/s10719-016-9705-z
Sakamoto H, Mashima T, Yamamoto K, Tsuruo T (2002) Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification. J Biol Chem 277:45770–45775. https://doi.org/10.1074/jbc.M207485200
Schalkwijk CG, van Bezu J, van der Schors RC, Uchida K, Stehouwer CD, van Hinsbergh VW (2006) Heat-shock protein 27 is a major methylglyoxal-modified protein in endothelial cells. FEBS Lett 580:1565–1570. https://doi.org/10.1016/j.febslet.2006.01.086
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354
van Heijst JW, Niessen HW, Musters RJ, van Hinsbergh VW, Hoekman K, Schalkwijk CG (2006) Argpyrimidine-modified heat shock protein 27 in human non-small cell lung cancer: a possible mechanism for evasion of apoptosis. Cancer Lett 241:309–319. https://doi.org/10.1016/j.canlet.2005.10.042
Wang Y, Kuramitsu Y, Tokuda K, Okada F, Baron B, Akada J, Kitagawa T, Nakamura K (2014) Proteomic analysis indicates that overexpression and nuclear translocation of lactoylglutathione lyase (GLO1) is associated with tumor progression in murine fibrosarcoma. Electrophoresis 35:2195–2202. https://doi.org/10.1002/elps.201300497
Weeks SD, Muranova LK, Heirbaut M, Beelen S, Strelkov SV, Gusev NB (2018) Characterization of human small heat shock protein HSPB1 alpha-crystallin domain localized mutants associated with hereditary motor neuron diseases. Sci Rep 8:688. https://doi.org/10.1038/s41598-017-18874-x
Zhou YH, Chen Z, Purcell RH, Emerson SU (2007) Positive reactions on Western blots do not necessarily indicate the epitopes on antigens are continuous. Immunol Cell Biol 85:73–78. https://doi.org/10.1038/sj.icb.7100004
Acknowledgements
The authors are grateful to Dr. Marina V. Serebryakova (A.N. Belozersky Institute of Physico-Chemical Biology, Moscow State University, Moscow 119991, Russian Federation) for performing mass spectroscopy experiments. The MALDI MS facility became available in the framework of the Moscow State University Development Program PNG 5.13.
Funding
This investigation was supported by the Russian Foundation for Basic Science (16-04-00016, 19-04-00038).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Figure S1
(PNG 213 kb)
High resolution image
(TIF 379 kb)
Figure S2
(PNG 216 kb)
High resolution image
(TIF 361 kb)
Figure S3
(PNG 165 kb)
High resolution image
(TIF 294 kb)
Rights and permissions
About this article
Cite this article
Sudnitsyna, M.V., Gusev, N.B. Is the small heat shock protein HspB1 (Hsp27) a real and predominant target of methylglyoxal modification?. Cell Stress and Chaperones 24, 419–426 (2019). https://doi.org/10.1007/s12192-019-00975-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-019-00975-3