Abstract
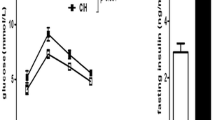
Increasing studies have shown protective effects of intermittent hypoxia on brain injury and heart ischemia. However, the effect of intermittent hypoxia on blood glucose metabolism, especially in diabetic conditions, is rarely observed. The aim of this study was to investigate whether intermittent hypoxia influences blood glucose metabolism in type 1 diabetic rats. Streptozotocin-induced diabetic adult rats and age-matched control rats were treated with intermittent hypoxia (at an altitude of 3 km, 4 h per day for 3 weeks) or normoxia as control. Fasting blood glucose, body weight, plasma fructosamine, plasma insulin, homeostasis model assessment of insulin resistance (HOMA-IR), pancreas β-cell mass, and hepatic and soleus glycogen were measured. Compared with diabetic rats before treatment, the level of fasting blood glucose in diabetic rats after normoxic treatment was increased (19.88 ± 5.69 mmol/L vs. 14.79 ± 5.84 mmol/L, p < 0.05), while it was not different in diabetic rats after hypoxic treatment (13.14 ± 5.77 mmol/L vs. 14.79 ± 5.84 mmol/L, p > 0.05). Meanwhile, fasting blood glucose in diabetic rats after hypoxic treatment was also lower than that in diabetic rats after normoxic treatment (13.14 ± 5.77 mmol/L vs. 19.88 ± 5.69 mmol/L, p<0.05). Plasma fructosamine in diabetic rats receiving intermittent hypoxia was significantly lower than that in diabetic rats receiving normoxia (1.28 ± 0.11 vs. 1.39 ± 0.11, p < 0.05), while there were no significant changes in body weight, plasma insulin and β-cell mass. HOMA-IR in diabetic rats after hypoxic treatment was also lower compared with diabetic rats after normoxic treatment (3.48 ± 0.48 vs. 3.86 ± 0.42, p < 0.05). Moreover, intermittent hypoxia showed effect on the increase of soleus glycogen but not hepatic glycogen. We conclude that intermittent hypoxia maintains glycemia in streptozotocin-induced diabetic rats and its regulation on muscular glycogenesis may play a role in the underlying mechanism.




Similar content being viewed by others
References
Anderson J, Honigman B (2011) The effect of altitude-induced hypoxia on heart disease: do acute, intermittent, and chronic exposures provide cardioprotection? High Alt Med Biol 12:45–55
Azevedo JJ, Carey J, Pories W, Morris P, Dohm G (1995) Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes 44:695–698
Brooks G et al (1991) Increased dependence on blood glucose after acclimatization to 4,300 m. J Appl Physiol 70:919–927
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Burtscher M et al (2009) Intermittent hypoxia increases exercise tolerance in patients at risk for or with mild COPD. Respir Physiol Neurobiol 165:97–103
Cartee G, Douen A, Ramlal T (1991) Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol 70:1593–1600
DeFronzo R, Jacot E, Jequier E, Maeder E, Wahren J, Felber J (1981) The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30:1000–1007
Dill R, Chadan S, Li C, Parkhouse W (2001) Aging and glucose transporter plasticity in response to hypobaric hypoxia. Mech Ageing Dev 122:533–545
Drager L, Jun J, Polotsky V (2010) Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab 24:843–851
Finegood D, McArthur M, Kojwang D, Thomas M, Topp B, Leonard T, Buckingham R (2001) Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes 50:1021–1029
Galle A, Jones N (2012) The neuroprotective actions of hypoxic preconditioning and postconditioning in a neonatal rat model of hypoxic-ischemic brain injury. Brain Res. doi:10.1016/j.brainres.2012.12.026
Gamboa J, Garcia-Cazarin M, Andrade F (2011) Chronic hypoxia increases insulin-stimulated glucose uptake in mouse soleus muscle. Am J Physiol Regul Integr Comp Physiol 300:R85–R91
Hamamoto Y et al (2001) Recovery of function and mass of endogenous beta-cells in streptozotocin-induced diabetic rats treated with islet transplantation. Biochem Biophys Res Commun 287:104–109
Holmes B, Kurth-Kraczek E, Winder W (1999) Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol 87:1990–1995
Klein R, Klein B, Moss S (1996) Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med 124:90–96
Lal C, Strange C, Bachman D (2012) Neurocognitive impairment in obstructive sleep apnea. Chest 141:1601–1610
Lee E et al (2013) Time-dependent changes in glucose and insulin regulation during intermittent hypoxia and continuous hypoxia. Eur J Appl Physiol 113:467–478
Lindgärde F, Ercilla M, Correa L, Ahrén B (2004) Body adiposity, insulin, and leptin in subgroups of Peruvian Amerindians. High Alt Med Biol 5:27–31
Lippl F, Neubauer S, Schipfer S, Lichter N, Tufman A, Otto B, Fischer R (2010) Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity (Silver Spring) 18:675–681
Liu J et al (2012) Intrauterine growth retardation increases the susceptibility of pigs to high-fat diet-induced mitochondrial dysfunction in skeletal muscle. PLoS One 7:e34835
Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P (2011) Acute hypoxia and exercise improve insulin sensitivity (S(I) (2*)) in individuals with type 2 diabetes. Diabetes Metab Res Rev 27:94–101
Mackenzie R, Maxwell N, Castle P, Elliott B, Brickley G, Watt P (2012) Intermittent exercise with and without hypoxia improves insulin sensitivity in individuals with type 2 diabetes. J Clin Endocrinol Metab 97:E546–E555
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
McCoy M, Proietto J, Hargreaves M (1996) Skeletal muscle GLUT-4 and postexercise muscle glycogen storage in humans. J Appl Physiol 80:411–415
Perseghin G et al (1996) Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335:1357–1362
Pescador N et al (2010) Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS One 5:e9644
Prabhakar N, Semenza G (2012) Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 92:967–1003
Rafacho A, Gonçalves-Neto L, Ferreira F, Protzek A, Boschero A, Nunes E, Zoccal D (2013) Glucose homoeostasis in rats exposed to acute intermittent hypoxia. Acta Physiol (Oxf). doi:10.1111/apha.12118
Roberts A, Reeves J, Butterfield G, Mazzeo R, Sutton J, Wolfel E, Brooks G (1996) Altitude and beta-blockade augment glucose utilization during submaximal exercise. J Appl Physiol 80:605–615
Roglic G et al (2005) The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 28:2130
Ruzzin J, Jensen J (2005) Contraction activates glucose uptake and glycogen synthase normally in muscles from dexamethasone-treated rats. Am J Physiol Endocrinol Metab 289:E241–E250
**a Y, Warshaw J, Haddad G (1997) Effect of chronic hypoxia on glucose transporters in heart and skeletal muscle of immature and adult rats. Am J Physiol 273:1734–1741
Youn J, Gulve E, Holloszy J (1991) Calcium stimulates glucose transport in skeletal muscle by a pathway independent of contraction. Am J Physiol 260:C555–C561
Young A, Evans W, Cymerman A, Pandolf K, Knapik J, Maher J (1982) Sparing effect of chronic high-altitude exposure on muscle glycogen utilization. J Appl Physiol 52:857–862
Yu S et al (2008) Hypoxic preconditioning up-regulates glucose transport activity and glucose transporter (GLUT1 and GLUT3) gene expression after acute anoxic exposure in the cultured rat hippocampal neurons and astrocytes. Brain Res 1211:22–29
Zhang Z, Lian B, Cui F (2008) Effect of FeSO4 treatment on glucose metabolism in diabetic rats. Biometals 21:685–691
Zhou J et al (2009) Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol 606:262–268
Zhu L et al (2005) Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res 1055:1–6
Zhu X et al (2010) Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci 30:12653–12663
Acknowledgments
This study was supported by the National Basic Research Programs of China (2011CB910800 and 2012CB518200).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All treatments were approved by the Institutional Animal Care and Use Committee of the Academy of Military Medical Sciences.
Rights and permissions
About this article
Cite this article
Chen, X., Zhao, T., Huang, X. et al. Intermittent hypoxia maintains glycemia in streptozotocin-induced diabetic rats. Cell Stress and Chaperones 21, 515–522 (2016). https://doi.org/10.1007/s12192-016-0679-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-016-0679-3