Abstract
Background
Although the chemotherapy-induced depletion of circulating white blood cells (WBC) is well recognized, the impact of exclusive radiotherapy (RT) on the different subpopulations of WBC remains unexplored. This may be important for immunotherapy administrated in combination with radiation, especially in malignant tumors usually treated with RT or chemoradiotherapy (CRT), and characterized by a high mutational burden, such as endometrial (EC) or cervical cancer (CC). We aimed to evaluate the impact of RT and CRT on circulating WBC in uterine cancers and its correlation with survival.
Material and methods
A total of 202 consecutive patients with uterine cancers treated with RT or CRT between 2009 and 2016 in a large European center and with available basal and post-treatment blood tests were retrospectively evaluated. EC and CC patients were analyzed separately. The differences between pre- and post- treatment WBC mean values were evaluated independently in patients treated with CRT and exclusive RT. Two-sided T test for paired samples and Kaplan–Meier curves were applied for analysis (p value < 0.05, SPSS v.23).
Results
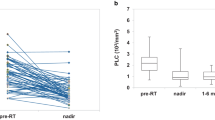
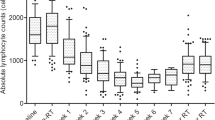
Among EC patients, 29 received CRT and 34 exclusive postoperative RT, while in CC cohort, 105 were treated with CRT and 34 with RT. In both cohorts, CRT affected significantly all WBC subtypes, whereas exclusive RT decreased only lymphocytes population (p = 0.000). Radiation-induced lymphopenia (RIL) had no impact on survival outcomes.
Conclusions
The selective depletion of lymphocytes after RT was significant in both EC and CC. Our results are of interest for further research on RIL and for design of immunotherapy-based clinical trials.


Similar content being viewed by others
References
Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51. https://doi.org/10.1016/j.critrevonc.2018.01.003.
Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015;13:1225–311.
Yamagata S, Green GH. Radiation-induced immune changes in patients with cancer of the cervix. Br J Obstet Gynaecol. 1976;83(5):400–8.
Standish LJ, Torkelson C, Hamill FA, et al. Immune defects in breast cancer patients after radiotherapy. J Soc Integr Oncol. 2008;6(3):110–21.
Schad MD, Dutta SW, Muller DM, et al. Radiation-related lymphopenia after pelvic nodal irradiation for prostate cancer. Adv Radiat Oncol. 2019;4(2):323–30. https://doi.org/10.1016/j.adro.2019.01.005. (eCollection 2019 Apr-Jun)
Ellsworth SG, Yalamanchali A, Zhang H, et al. Comprehensive analysis of the kinetics of radiation-induced lymphocyte loss in patients treated with external beam radiation therapy. Radiat Res. 2020;193(1):73–81. https://doi.org/10.1667/RR15367.1(Epub 2019 Nov 1).
Herzog T, Arguello D, Reddy S, et al. PD-1 and PD-L1 expression in 1599 gynecological malignancies: implications for immunotherapy. Gynecol Oncol. 2015;137:204–5.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590(Epub 2020 Jan 8).
Grywalska E, Sobstyl M, Putowski L, et al. Current possibilities of gynecologic cancer treatment with the use of immune checkpoint inhibitors. Int J Mol Sci. 2019;20(19):4705. https://doi.org/10.3390/ijms20194705.
Kagabu M, Nagasawa T, Fukagawa D, et al. Immunotherapy for uterine cervical cancer. Healthcare (Basel). 2019;7(3):108. https://doi.org/10.3390/healthcare7030108.
Lynam S, Lugade AA, Odunsi K. Immunotherapy for gynecologic cancer: current applications and future directions. Clin Obstet Gynecol. 2019. https://doi.org/10.1097/GRF.0000000000000513.
Rubinstein MM, Makker V. Optimizing immunotherapy for gynecologic cancers. Curr Opin Obstet Gynecol. 2020;32(1):1–8. https://doi.org/10.1097/GCO.0000000000000603.
Wu ES, Oduyebo T, Cobb LP, et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol. 2016;140(1):76–82. https://doi.org/10.1016/j.ygyno.2015.11.013(Epub 2015 Nov 11).
Diehl A, Yarchoan M, Hopkins A, Jaffee EM, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8:114268.
Zhuang Y, Yuan BY, Chen GW, et al. Association between circulating lymphocyte populations and outcome after stereotactic body radiation therapy in patients with hepatocellular carcinoma. Front Oncol. 2019;9:896. https://doi.org/10.3389/fonc.2019.00896(eCollection 2019).
Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89(5):1084–91. https://doi.org/10.1016/j.ijrobp.2014.04.025.
van Rossum PSN, Deng W, Routman DM. Prediction of Severe Lymphopenia During Chemoradiation Therapy for Esophageal Cancer: Development and Validation of a Pretreatment Nomogram. Pract Radiat Oncol. 2020;10(1):e16–e26. https://doi.org/10.1016/j.prro.2019.07.010(Epub 2019 Jul 29).
Chadha AS, Liu G, Chen HC, et al. Does unintentional splenic radiation predict outcomes after pancreatic cancer radiation therapy? Int J Radiat Oncol Biol Phys. 2017;97(2):323–32.
Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–65.
Ng SP, Bahig H, Jethanandani A, et al. Lymphopenia during radiotherapy in patients with oropharyngeal cancer. Radiother Oncol. 2020;145:95–100. https://doi.org/10.1016/j.radonc.2019.12.023.
Cho O, Oh YT, Chun M, et al. Radiation-related lymphopenia as a new prognostic factor in limited-stage small cell lung cancer. Tumour Biol. 2016;37(1):971–8. https://doi.org/10.1007/s13277-015-3888-y(Epub 2015 Aug 12).
Ho WJ, Yarchoan M. Hopkins A et al Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J Immunother Cancer. 2018;6(1):84. https://doi.org/10.1186/s40425-018-0395-x.
Soyano AE, Dholaria B, Marin-Acevedo JA, et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung cancer patients treated with anti-PD-1 antibodies. J Immunother Cancer. 2018;6(1):129. https://doi.org/10.1186/s40425-018-0447-2.
** JY, Mereniuk T, Yalamanchali A, et al. A framework for modeling radiation induced lymphopenia in radiotherapy. Radiother Oncol. 2019;144:105–13. https://doi.org/10.1016/j.radonc.2019.11.014.
Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903.
Sun R, Champiat S, Dercle L, et al. Baseline lymphopenia should not be used as exclusion criteria in early clinical trials investigating immune checkpoint blockers (PD-1/PD-L1 inhibitors). Eur J Cancer. 2017;84:202–11. https://doi.org/10.1016/j.ejca.2017.07.033(Epub 2017 Aug 18).
Deng W, Xu C, Liu A, et al. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother Oncol. 2019;133:9–15. https://doi.org/10.1016/j.radonc.2018.12.002(Epub 2019 Jan 11).
Tsuchiya T, Someya M, Takada Y, et al. Association between radiotherapy-induced alteration of programmed death ligand 1 and survival in patients with uterine cervical cancer undergoing preoperative radiotherapy. Strahlenther Onkol. 2020. https://doi.org/10.1007/s00066-019-01571-1.
Dovšak T, Ihan A, Didanovič V, et al. Effect of surgery and radiotherapy on complete blood count, lymphocyte subsets and inflammatory response in patients with advanced oral cancer. BMC Cancer. 2018;18(1):235. https://doi.org/10.1186/s12885-018-4136-9.
Acknowledgements
The authors would like to thank Mrs. Rosa Gimenez for her assistance.
Funding
This study received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in our studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Some preliminary results of this study were accepted for the presentation at the 39th ESTRO Annual Meeting in 2020 in Vienna.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Holub, K., Vargas, A. & Biete, A. Radiation-induced lymphopenia: the main aspects to consider in immunotherapy trials for endometrial and cervical cancer patients. Clin Transl Oncol 22, 2040–2048 (2020). https://doi.org/10.1007/s12094-020-02345-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02345-3