Abstract
Background
Data regarding the real-world effectiveness and safety of sofosbuvir/velpatasvir (SOF/VEL) for East Asian patients with chronic hepatitis C virus (HCV) infection and compensated liver disease are limited. We evaluated the performance of SOF/VEL for 12 weeks for HCV-infected patients with compensated liver disease in a large real-world cohort in Taiwan.
Methods
Between July 2019 and March 2020, 1880 HCV-infected patients with compensated liver disease who received SOF/VEL 400/100 mg once daily for 12 weeks were included at 15 academic centers in Taiwan. The sustained virologic response at off-treatment week 12 (SVR12) was assessed for evaluable (EP) and per-protocol populations (PP). The tolerance was also reported.
Results
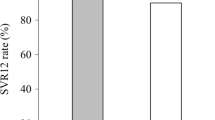
The SVR12 rates by EP and PP analyses were 95.6% [1798 of 1880 patients; 95% confidence interval (CI) 94.6–96.5%] and 99.3% (1798 of 1811 patients; 95% CI 98.8–99.6%), respectively. Among 82 patients who failed to achieve SVR12, 13 (15.9%) were attributed to virologic failures. The SVR12 rates were comparable regardless of baseline characteristics. A total of 1859 (98.9%) patients completed 12-week SOF/VEL treatment. Four (0.2%) patients discontinued treatment due to adverse events (AEs). All patients with serious AEs or deaths were judged not related to SOF/VEL. The AEs occurring in ≥ 10% included headache (16.8%), fatigue (16.2%), nausea (11.8%), and insomnia (11.1%). Nine (0.5%) and 2 (0.1%) patients had grade 3 total bilirubin and alanine aminotransferase elevations.
Conclusions
SOF/VEL for 12 weeks is efficacious and well-tolerated by chronic HCV-infected patients with compensated liver disease in Taiwan.


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Abbreviations
- HCV:
-
Hepatitis C virus
- HCC:
-
Hepatocellular carcinoma
- IFN:
-
Interferon
- DAA:
-
Direct acting antiviral
- SOF:
-
Sofosbuvir
- VEL:
-
Velpatasvir
- SVR:
-
Sustained virologic response
- HBV:
-
Hepatitis B virus
- HIV:
-
Human immunodeficiency virus
- FDC:
-
Fixed-dose combination
- GT:
-
Genotype
- DDI:
-
Drug–drug interaction
- PI:
-
Protease inhibitor
- LLOQ:
-
Lower limit of quantification
- ULN:
-
Upper limit of normal
- eGFR:
-
Estimated glomerular filtration rate
- RNA:
-
Ribonucleic acid
- DNA:
-
Deoxyribonucleic acid
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- FIB-4:
-
Fibrosis index based on 4 parameters
- APRI:
-
Aspartate aminotransferase-to-platelet ratio index
- AE:
-
Adverse event
- IQR:
-
Interquartile range
- CI:
-
Confidence interval
- RBV:
-
Ribavirin
- RAS:
-
Resistance-associated substitution
References
Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161–176
Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, R.E.V.E.A.L.-HCV Study Group, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis 2012;206:469–477
Younossi ZM, Henry L, Pong J, Tanaka A, Eguchi Y, Mizokami M, et al. Systematic review with meta-analysis: extrahepatic manifestations in chronic hepatitis C virus-infected patients in East Asia. Aliment Pharmacol Ther 2019;49:644–653
Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, French ANRS CO22 Hepather cohort, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 2019;393:1453–1464
AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis 2018;67:1477–1492
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol 2020;73:1170–1218
Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int 2016;10:702–726
Greig SL. Sofosbuvir/velpatasvir: a review in chronic hepatitis C. Drugs 2016;76:1567–1578
Gottwein JM, Pham LV, Mikkelsen LS, Ghanem L, Ramirez S, Scheel TKH, et al. Efficacy of NS5A inhibitors against hepatitis C virus genotypes 1–7 and escape variants. Gastroenterology 2018;154:1435–1448
Liu CH, Yu ML, Peng CY, Hsieh TY, Huang YH, Su WW, et al. Comorbidities, concomitant medications, and potential drug-drug interactions with interferon-free direct-acting antiviral agents in hepatitis C patients in Taiwan. Aliment Pharmacol Ther 2018;48:1290–1300
Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, ASTRAL-1 Investigators, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015;373:2599–2607
Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, ASTRAL-2 Investigators; ASTRAL-3 Investigators, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015;373:2608–2617
Wei L, Lim SG, **e Q, Văn KN, Piratvisuth T, Huang Y, et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol 2019;4:127–134
Mangia A, Milligan S, Khalili M, Fagiuoli S, Shafran SD, Carrat F, et al. Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: analysis of 5552 patients from 12 cohorts. Liver Int 2020;40:1841–1852
Li JP, Chen XF, Yan Q, Zhang YJ, **e ZW, **a Y, et al. Effectiveness and safety of sofosbuvir/velpatasvir combination ± ribavirin in the treatment of Chinese adults with chronic hepatitis C virus infection. Zhonghua Gan Zang Bing Za Zhi 2020;28:831–837
Liu CH, Sun HY, Liu CJ, Sheng WH, Hsieh SM, Lo YC, et al. Generic velpatasvir plus sofosbuvir for hepatitis C virus infection in patients with or without human immunodeficiency virus coinfection. Aliment Pharmacol Ther 2018;47:1690–1698
Liu CH, Huang YJ, Yang SS, Chang CH, Yang SS, Sun HY, et al. Generic sofosbuvir-based interferon-free direct acting antiviral agents for patients with chronic hepatitis C virus infection: a real-world multicenter observational study. Sci Rep 2018;8:13699
Hu C, Yuan G, Liu J, Huang H, Ren Y, Li Y, et al. Sofosbuvir-based therapies for patients with hepatitis C virus infection: real-world experience in China. Can J Gastroenterol Hepatol 2018;2018:3908767
Liu CH, Lee MH, Lin JW, Liu CJ, Su TH, Tseng TC, et al. Evolution of eGFR in chronic HCV patients receiving sofosbuvir-based or sofosbuvir-free direct-acting antivirals. J Hepatol 2020;72:839–846
Liu CH, Liang CC, Liu CJ, Lin CL, Su TH, Yang HC, et al. Comparison of Abbott RealTime HCV genotype II with versant line probe assay 2.0 for hepatitis C virus genoty**. J Clin Microbiol 2015;53:1754–1757
Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, APRICOT Clinical Investigators, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325
Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–526
Hezode C, Reau N, Svarovskaia ES, Doehle BP, Shanmugam R, Dvory-Sobol H, et al. Resistance analysis in patients with genotype 1–6 HCV infection treated with sofosbuvir/velpatasvir in the phase III studies. J Hepatol 2018;68:895–903
Poordad F, Nelson DR, Feld JJ, Fried MW, Wedemeyer H, Larsen L, et al. Safety of the 2D/3D direct-acting antiviral regimen in HCV-induced Child-Pugh A cirrhosis: a pooled analysis. J Hepatol 2017;67:700–707
Sidharthan S, Kohli A, Sims Z, Nelson A, Osinusi A, Masur H, et al. Utility of hepatitis C viral load monitoring on direct-acting antiviral therapy. Clin Infect Dis 2015;60:1743–1751
Izumi N, Takehara T, Chayama K, Yatsuhashi H, Takaguchi K, Ide T, et al. Sofosbuvir-velpatasvir plus ribavirin in Japanese patients with genotype 1 or 2 hepatitis C who failed direct-acting antivirals. Hepatol Int 2018;12:356–367
Sood A, Duseja A, Kabrawala M, Amrose P, Goswami B, Chowdhury A, et al. Sofosbuvir-velpatasvir single-tablet regimen administered for 12 weeks in a phase 3 study with minimal monitoring in India. Hepatol Int 2019;13:173–179
Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol 2017;67:1204–1212
Chugh Y, Dhiman RK, Premkumar M, Prinja S, Singh GG, Bahuguna P. Real-world cost-effectiveness of pan-genotypic sofosbuvir-velpatasvir combination versus genotype dependent directly acting anti-viral drugs for treatment of hepatitis C patients in the universal coverage scheme of Punjab state in India. PLoS One 2019;14:e0221769
Liu CH, Liu CJ, Su TH, Fang YJ, Yang HC, Chen PJ, et al. Hepatitis B virus reactivation in patients receiving interferon-free direct-acting antiviral agents for chronic hepatitis C virus infection. Open Forum Infect Dis 2017;4:ofx028
Acknowledgements
The authors thank Hui-Ju Lin and Pin-Chin Huang for clinical data management; the 7th Core Lab of National Taiwan University Hospital and the 1st Common Laboratory of National Taiwan University Hospital, Yun-Lin Branch for instrumental and technical support.
Funding
The study was supported by Ministry of Science and Technology, Taiwan (106-2314-B-002-138-MY3, 107-2314-B-002-038-MY2).
Author information
Authors and Affiliations
Contributions
Conception and design: CHL and JHK. Analysis and interpretation of data: CHL. Drafting of the article: CHL and JHK. Critical revision of the article for important intellectual content: CHL, PYC, JJC, CCL, WWS, KCT, CJL, CSH, KJH, SSY, CYP, MCT, WYK, CYC, YLS, YJF, CYC, PLL, JJH, PYS, CWT, CCH, CHC, YJH, HCL, CCC, FJL, TYH, and JHK. Final approval of the article: CHL, PYC, JJC, CCL, WWS, KCT, CJL, CSH, KJH, SSY, CYP, MCT, WYK, CYC, YLS, YJF, CYC, PLL, JJH, PYS, CWT, CCH, CHC, YJH, HCL, CCC, FJL, TYH, and JHK. Provision of study materials or patients: CHL, PYC, JJC, CCL, WWS, KCT, CJL, CSH, KJH, SSY, CYP, MCT, WYK, CYC, YLS, YJF, CYC, PLL, JJH, PYS, CWT, CCH, CHC, YJH, HCL, CCC, FJL, TYH, and JHK. Statistical expertise: CH Liu. Administrative, technical, or logistic support: CHL and JHK. Collection and assembly of data: CHL.
Corresponding author
Ethics declarations
Conflict of interest
Chen-Hua Liu: advisory board for Abbvie, Gilead Sciences, Merck Sharp & Dohme; speaker’s bureau for Abbott, Abbvie, Gilead Sciences, Merck Sharp & Dohme; research grant from Abbvie, Gilead Science, Merck Sharp & Dohme. Sheng-Shun Yang: advisory board for Abbvie, Roche, Ipsen; speaker’s bureau for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Ipsen, Merck Sharp & Dohme. Chien-Ching Hung: advisory board for Abbvie, Gilead Sciences, ViiV Healthcare; speaker’s bureau for Gilead Sciences; research grant from Gilead Sciences, ViiV Healthcare, Merck Sharp & Dohme. Jia- Horng Kao: advisory board for Abbott, Abbvie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Roche; speaker’s bureau for Abbott, Abbvie, Bayer, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, and Roche. All other authors declare no competing interests.
Ethical approval
The study was approved by the Research Ethics Committee of each participating center (ID: 202007043RIND) and was conducted in accordance with the principles of Declaration of Helsinki in 1975.
Animal research
This was not an animal research.
Consent to participate
This was a retrospective study with clinical data collection, and the Research Ethics Committee of each participating center approved to waive the patient consent.
Consent to publish
All the authors consented the publish work.
Clinical trials registration
The was a retrospective observation study and was not a drug trial. There was no need for clinical trial registration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12072_2021_10158_MOESM1_ESM.pdf
Supplementary file1 Supplementary Figure 1. eGFR changes between baseline and SVR12 according to baseline CKD stage. (PDF 23 KB)
Rights and permissions
About this article
Cite this article
Liu, CH., Chen, PY., Chen, JJ. et al. Sofosbuvir/velpatasvir for patients with chronic hepatitis C virus infection and compensated liver disease: real-world data in Taiwan. Hepatol Int 15, 338–349 (2021). https://doi.org/10.1007/s12072-021-10158-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10158-x