Abstract
Epoxy thermoset polymer materials (P1, P2, and P3) using resorcinol diglycidyl ether (RE) with different primary amines having aliphatic and aromatic backbones were prepared without using any transition metal-based catalyst and solvent. The polymerization was carried out with comonomers in the presence of non-nucleophilic base 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and CO2 gas through bulk copolymerization within 10-15 min at 100 °C yielding the cross-linked polymer. This cross-linking reaction can capture CO2 while forming a thermoset. The ring-strain-induced reactivity of oxiranes and the proton-removing ability of the sterically hindered non-nucleophilic base have driven the copolymerization reactions. Considering the fast pace in which the reaction occurred and the material’s hardness, the method reported here has the potential for large-scale industrial application. The resultant epoxy thermosets showed high glass transition temperature (Tg) in the range of 67–106 °C and possessed hardness up to 24.26 HV in the Vickers microhardness scale.
Graphical abstract
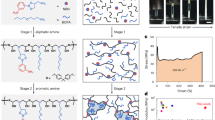
Epoxy thermoset polymer materials are prepared from resorcinol diglycidyl ether (RE) reactions with different primary amines. These materials capture carbon dioxide rapidly during the cross-linking reactions yielding highly abrasive materials as determined by the Vickers microhardness tester.







Similar content being viewed by others
References
Briscoe B J and Sinha S K 1999 Hardness and Normal Indentation of Polymers In Mechanical Properties and Testing of Polymers Vol. 3 Polymer Science and Technology Series G M Swallowe (Ed.) (Dordrecht: Springer)
(a) Soloukhin V A, Brokken-Zijp J C M, van Asselen O L J and de With G 2003 Physical Aging of Polycarbonate: Elastic Modulus, Hardness, Creep, Endothermic Peak, Molecular Weight Distribution, and Infrared Data Macromolecules 36 7585; (b) Crawford R J 1982 Microhardness testing of plastics Polym. Test. 3 37
Baltá Calleja F J and Fakirov S 2000 Microhardness of Polymers (Cambridge University Press)
(a) Guo Q 2017 Thermosets - Structure, Properties, and Applications 2nd edn. (Amsterdam: Elsevier); (b) Streitberger H–J and Dössel K–F (Eds.) 2008 Automotive Paints and Coatings 2nd edn. (Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA); (c) Irfan M H 1998 Chemistry and Technology of Thermosetting Polymers in Construction Applications (Dordrecht, Netherlands: Springer) p.78
(a) Vandenberg L N, Hauser R, Marcus M, Olea N and Welshons W V 2007 Human exposure to bisphenol A (BPA) Reprod. Toxicol. 24 139; (b) Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A and Shimohigashi Y 2008 Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-γ Environ. Health Perspect. 116 32; (c) Vom Saal F S, Akingbemi B T, Belcher S M, Birnbaum L S, Crain D A Eriksen M, Farabollini, F, Guillette Jr. L J, Hauser R, Heindel J J et al. 2007 Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure Reprod. Toxicol. 24 131
(a) R A Meyers (Ed.) 2018 Conversion of CO2 into Polymers, Encyclopedia of Sustainability Science and Technology (New York: Springer); (b) B Metz, O Davidson, H C de Coninck, M Loos and L Meyer (Eds.) 2005 IPCC Special Report on Carbon Dioxide Capture and Storage. Prepared by Working Group III of the Intergovernmental Panel on Climate Change (IPCC) (Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press) p. 442
(a) Wu S, Zhang Y, Wang B, Elageed E H M, Ji L, Wu H and Gao G 2017 Synthesis of Functionalized Cyclic Carbonates by One-Pot Reactions of Carbon Dioxide, Epibromohydrin, and Phenols, Thiophenols, or Carboxylic Acids Catalysed by Ionic Liquids Eur. J. Org. Chem. 3 753; (b) Dalpozzo R, Della Ca N, Gabriele B and Mancuso R 2019 Recent Advances in the Chemical Fixation of Carbon Dioxide: A Green Route to Carbonylated Heterocycle Synthesis Catalysts 9 511; (c) Leung D Y C, Caramanna G and Maroto-Valer M 2014 An overview of current status of carbon dioxide capture and storage technologies Renew. Sustain. Energy Rev. 39 426
(a) Mikkelsen M, Jorgensen M and Krebs F C 2010 The teraton challenge. A review of fixation and transformation of carbon dioxide Energy Environ. Sci. 3 43; (b) North M, Pasquale R and Young C 2010 Synthesis of cyclic carbonates from epoxides and CO2 Green Chem. 12 1514
(a) Verma S, Kumar G, Ansari A, Kureshy R I and Khanab N H 2017 A nitrogen rich polymer as an organo-catalyst for cycloaddition of CO2 to epoxides and its application for the synthesis of polyurethane Sustain. Energy Fuels 1 1620; (b) Sun J and Kuckling D 2016 Synthesis of high-molecular-weight aliphatic polycarbonates by organo-catalysis Polym. Chem. 7 1642; (c) Bian S, Pagan C, Andrianova A, Artemyeva A and Du G 2016 Synthesis of Polycarbonates and Poly(ether carbonate)s Directly from Carbon Dioxide and Diols Promoted by a Cs2CO3/CH2Cl2 System ACS Omega 1 1049; (d) Ghosh S, Ghosh A, Biswas S, Sengupta M, Roy D and Islam Sk M 2019 Palladium Grafted Functionalized Nanomaterial: An Efficient Catalyst for the CO2 Fixation of Amines and Production of Organic Carbamates ChemistrySelect 4 3961; (e) Lambeth R H and Henderson T J 2013 Organocatalytic synthesis of (poly)hydroxyurethanes from cyclic carbonates and amines Polymer 54 5568; (f) Moore D R, Cheng M, Lobkovsky E B and Coates G W 2003 Mechanism of the Alternating Copolymerization of Epoxides and CO2 Using β-Diiminate Zinc Catalysts: Evidence for a Bimetallic Epoxide Enchainment J. Am. Chem. Soc. 125 11911; (g) Liu A H, Dang Y L, Zhou H, Zhang J J and Lu X-B 2018 CO2 Adducts of Carbodicarbenes: Robust and Versatile Organocatalysts for Chemical Transformation of Carbon Dioxide into Heterocyclic Compounds ChemCatChem 10 2686
(a) Babu H V and Muralidharan K 2014 Versatile metal complexes of 2,5-bis {N-(2,6-di isopropylphenyl) iminomethyl} pyrrole for epoxide–CO2 coupling and ring opening polymerization of ε-caprolactone RSC Adv. 4 6094; (b) Babu H V and Muralidharan K 2014 Polyethers with phosphate pendant groups by monomer activated anionic ring opening polymerization: Syntheses, characterization and their lithium-ion conductivities Polymer 55 83; (c) Babu H V and Muralidharan K 2013 Zn(II), Cd(II) and Cu(II) complexes of 2,5-bis{N-(2,6-diisopropylphenyl)iminomethyl}pyrrole: synthesis, structures and their high catalytic activity for efficient cyclic carbonate synthesis Dalton Trans. 42 1238
Thevenon A, Cyriac A, Myers D, White A J P, Durr C B and Williams C K 2018 Indium Catalysts for Low-Pressure CO2/Epoxide Ring-Opening Copolymerization: Evidence for a Mononuclear Mechanism J. Am. Chem. Soc. 140 6893
Schimpf V, Max J B, Stolz B, Heck B and Mülhaupt R 2019 Semicrystalline Non-Isocyanate Polyhydroxyurethanes as Thermoplastics and Thermoplastic Elastomers and XTheir Use in 3D Printing by Fused Filament Fabrication Macromolecules 52 320
Verma S, Kumar G, Ansari A, Kureshy R I and Khan N H 2017 A nitrogen rich polymer as an organo-catalyst for cycloaddition of CO2 to epoxides and its application for the synthesis of polyurethane Sustain. Energy Fuels 1 1620
Nguyen Q-B Nguyen N-H, de Anda A R, Nguyen V-H, Versace D-L, Langlois V, Naili S and Renard E 2020 Photocurable bulk epoxy resins based on resorcinol derivative through cationic polymerization J. Appl. Polym. Sci. 137 49051
Mattar N, de Anda A R, Vahabi H, Renard E and Langlois V 2020 Resorcinol-Based Epoxy Resins Hardened with Limonene and Eugenol Derivatives: From the Synthesis of Renewable Diamines to the Mechanical Properties of Biobased Thermosets ACS Sustain. Chem. Eng. 8 13064
Cantarutti C, Dinu R and Mija A 2020 Polyhydroxybutyrate Bioresins with High Thermal Stability by Cross-linking with Resorcinol Diglycidyl Ether Biomacromolecules 21 3447
Yu G, Masazumi T, Yoshinao N, Kenji N, Kimihito S and Keiichi T 2021 Direct synthesis of polycarbonate diols from atmospheric flow CO2 and diols without using dehydrating agents Green Chem. 23 5786
Lv M, Wang P, Yuan D and Yao Y 2017 Conversion of Carbon Dioxide into Oxazolidinones Mediated by Quaternary Ammonium Salt and DBU ChemCatChem 9 4451
Heldebrant D J, Jessop P G, Thomas C A, Eckert C A and Liotta C L 2005 The Reaction of 1,8-Diazabicylo[5.4.0]undec-7-ene (DBU) with Carbon Dioxide J. Org. Chem. 70 5335
(a) Li G, Chen J, Zhu D Y, Chen Y and **aa J B 2018 DBU-Catalyzed Selective N-Methylation and N-Formylation of Amines with CO2 and Polymethylhydrosiloxane Adv. Synth. Catal. 360 2364; (b) Mancuso R, Ziccarelli I, Pomelli C S, Cuocci C, Ca N D, Olivieri D, Carfagna C and Gabriele B 2020 Unprecedented cooperative DBU-CuCl2 catalysis for the incorporation of carbon dioxide into homopropargylic amines leading to 6-methylene-1,3-oxazin-2-ones J. Catal. 387 145
Sweileh B A, Al-Hiari Y M, Kailani M H and Mohammad H A 2010 Synthesis and Characterization of Polycarbonates by Melt Phase Interchange Reactions of Alkylene and Arylene Diacetates with Alkylene and Arylene Diphenyl Dicarbonates Molecules 15 3661
Thomas V, Francois T, Simone M, Agathe R and Ludwik L 2016 Control of reactions and network structures of epoxythermosets Prog. Polymer Sci. 62 126
Mathew M, Shenoy K and Ravishankar K S 2014 Vickers Hardness and Specific Wear Rate of Poly Propylene Reinforced PMMA Int. J. Sci. Study 2 71
Mina M, Michler G and Balta Calleja F 2009 Glass transition temperature and microhardness of compatible and incompatible elastomer/plastomer blends J. Bangladesh Acad. Sci. 33 15
(a) Weiler W 2019 The Relationship Between Vickers Hardness and Universal Hardness NASF Surface Technology White Papers 83 13; (b) Smith R L and Sandly G E 1992 An Accurate Method of Determining the Hardness of Metals, with Particular Reference to Those of a High Degree of Hardness Proc. Inst. Mech. Eng. 102 39
(a) Low I M and Shi C 1998 Vickers indentation responses of epoxy polymers J. Mater. Sci. Lett. 17 1181; (b) Narisawa I, Ishikawa M and Nonak T 1988 Kobunshi Ronbunshu 45 777
Acknowledgements
Authors thank UGC, India, for funding through the BSR fellowship (VRV), UGC JRF (SK), and CAS program. We thank Prof. Jai Prakash Gautam, SEST, University of Hyderabad, for hardness measurements. Vickers hardness tester was purchased under the PURSE grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Velpuri, V.R., Kumari, S. & Muralidharan, K. Rapid capture of flow carbon dioxide by hard Epoxy thermosets with the high glass transition temperature. J Chem Sci 135, 24 (2023). https://doi.org/10.1007/s12039-023-02139-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-023-02139-4