Abstract
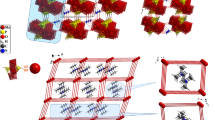
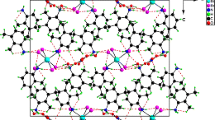
Two new inorganic-organic hybrid materials based on heteropolyoxometalates, (C4H10N)6(P2 Mo18O62).4H2O I, and (C4H10N)6(As2Mo18O62).4H2O II, where C4H10N is protonated pyrrolidine have been synthesized and structurally characterized by physic-chemical methods. Single-crystal X-ray diffraction method, infrared, ultraviolet spectroscopy, Thermogravimetricanalysis andcyclic voltammetry measurements of the title hybrid materials indicate that there are hydrogen bond interaction between O atoms of the heteropolyoxometalates and water molecules as well as the N and O atoms of the organic compound. The molecular structures of synthesized hybrid materials contain discrete entities of pyrrolidinumion and water molecules surround every [X2Mo18O62]6− anion over the extended crystalline network that the [X2Mo18O62]6− anion retains its “Dawson structure”. Crystal data: I monoclinic, space group P21/a, a = 13,453(1) Å, b = 24,046 (1) Å, c = 24,119(1) β = 97,99(1)°, V = 7726,30(5) Å3 and Z = 4; II monoclinic, space group P21/a, a = 13.4900(1) Å, 24.0900(1) Å, 24.2740(1) Å, β = 98.320(1)°, V = 7805.40(7) Å3 and Z = 4.

Two new inorganic-organic hybrid materials based on the inorganic cluster, [X2Mo18O62]6- (X = P, As), crystallize in monoclinic space group P21/a.






Similar content being viewed by others
References
Müller A, Peters F, Pope M T and Gatteschi D 1998 Chem. Rev. 98 239
Pope M T 1983 In Heteropoly and Isopoly Oxometalates (Springer: Berlin)
Gouzerh P and Proust A 1998 Chem. Rev. 98 77
Yamase T 1998 Chem. Rev. 98 307
Müller A, Shah S Q N, Bogge H and Schmidtmann M 1999 Nature 397 48
Rhule J T, Hill C L, Judd D A and Schinazi R F 1998 Chem. Rev. 98 327
Proust A, Thouvenot R and Gouzerh P 2008 Chem. Commun. 16 1837
Katsoulis D E 1998 Chem. Rev. 98 359
Kozhenikov I V 1998 Chem. Rev. 98 171
Razak I A, Raj S, Chantrapromma H-K, Fun Y-S and Zhou X-Z You 2001 J. Chem. Crystallogr. 31 255
Legagneux N, Jeanneau E, Basset J M and Lefebvre F 2009 J. Mol. Struct. 21 300
Ma H, Gong L, Wang F and Wang X 2008 Struct. Chem. 19 435
Dablemont C, Hamaker C G, Thouvenot R, Sojka Z, Che M, Maatta E A and Proust A 2006 Chem. Eur. J. 12 9150
Galan-Mascaros J R, Gimenez-Saiz C, Triki S, Gomez-Garcia C J, Coronado E and Ouahab L 1995 Angew. Chem. Int. Ed. 34 1460
An H Y, Lan Y, Li Y G, Wang E B, Hao N, **ao D R, Duan L Y and Xu L 2004 Inorg. Chem. Commun. 7 356
Li L C, Liao D Z, Jiang Z H and Yan S P 2002 Inorg. Chem. 41 1019
(a) Reinoso S, Vitoria P, Lezama L, Luque A and Gutiérrez- Zorrilla J M 2003 Inorg. Chem. 42 3709; (b) Lu Y, Xu Y, Wang E B, Lu J, Hu C W and Xu L 2005 Cryst. Growth Des. 5 257; (c) Bonhomme F, Larentzos J P, Alam T M, Maginn E J and Nyman M 2005 Inorg. Chem. 44 1774; (d) Vasylyeva M, Popovitz-Birob R, Shimonc L J W and Neumanna R 2003 J. Mol. Struct. 656 27
(a) Niu J Y, Wei M L, Wang J P and Dang D B 2004 Eur. J. Inorg. Chem. 1 160; (b) Wang J P, Zhao J W and Niu J Y 2004 J. Mol. Struct. 697 191
Hagrman P J, Hagrman D and Zubieta J 1999 Angew. Chem. Int. Ed. 38 3165
CAD-4 Express Software, Enraf-Nonius, Delft (1994) The Netherlands
Sheldrick G M, SHELXS97 and SHELXL97 (1997) Program for Crystal Structure Solution and Refinement (University of Goettingen: Goettingen, Germany)
Wang J P, Zhao J W and Niu J Y 2004 J. Mol. Struct. 697 191
Yu H, Zhang X, Kong L and Xu J 2009 Acta Cryst. E65 m1698
Soumahoro T, Burkholder E, Ouellette W and Zubieta J 2005 Inorg. Chim. Acta 358 606
**ghua L, Jun P, Enbo W, Lihua B and Shurong G 2000 J. Mol. Struct. 525 71
Pope M T 1976 Inorg. Chem. 15 2068
D’Amour H and Allmann R 1974 Naturwiss. 61 34
Strandberg R 1975 Acta Chem. Scand. A 29 350
Yang Y, Xu L, Jia L, Gao G, Li F, Qu X and Qiu Y 2007 Cryst. Res. Technol. 42 10
Brown I and Altermatt D 1985 Acta Crystallogr. Sect. B 4 1244
Pope M T and Papaconstantinou E 1967 Inorg. Chem. 6 1147
Liu D, Tan H Q, Chen W L, Li Y G and Wang E B 2010 Cryst. Eng. Comm. 12 2044
Acknowledgments
The crystal data collection of I and II was done in the Laboratoire de MateriauxetCristallochimie, Faculté des Sciences, El Manar, 2092, Tunis, Tunisia. We are grateful to Ahmed Driss who supervised this experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
The cif and checkCIF files for X-ray crystallography of the both compounds are available in supplementary information at www.ias.ac.in/chemsci. Crystallographic data for the structures of I and II have been deposited in the Cambridge Crystallographic Data center as publication number CCDC 1031359 and 1031358, respectively. Copy of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB21EZ, UK (fax: (+ 44) 1223-336-033; e-mail: deposit@ccdc.com.ac.uk).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
HMIDA, F., AYED, M., AYED, B. et al. Two new inorganic-organic hybrid materials based on inorganic cluster, [X2Mo18O62]6− (X=P, As). J Chem Sci 127, 1645–1651 (2015). https://doi.org/10.1007/s12039-015-0931-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0931-x