Abstract
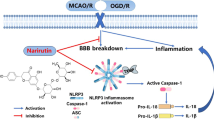
Ischemia–reperfusion (I/R) injury is a key influencing factor in the outcome of stroke. Inflammatory response, oxidative stress, and neuronal apoptosis are among the main factors that affect the progression of I/R injury. Farrerol (FAR) is a natural compound that can effectively inhibit the inflammatory response and oxidative stress. However, the role of FAR in cerebral I/R injury remains unknown. In this study, we found that FAR reduced brain injury and neuronal viability after cerebral I/R injury. Meanwhile, administration of FAR also reduced the inflammatory response of microglia after brain injury. Mechanistically, FAR treatment directly reduced neuronal death after oxygen glucose deprivation/re-oxygenation (OGD/R) through enhancing cAMP-response element binding protein (CREB) activation to increase the expression of downstream neurotrophic factors and anti-apoptotic genes. Moreover, FAR decreased the activation of nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways, inhibited microglia activation, and reduced the production of inflammatory cytokines in microglia after OGD/R treatment or LPS stimulation. The compromised inflammatory response by FAR directly promoted the survival of neurons after OGD/R. In conclusion, FAR exerted a protective effect on cerebral I/R injury by directly decreasing neuronal death through upregulating CREB expression and attenuating neuroinflammation. Therefore, FAR could be a potentially effective drug for the treatment of cerebral I/R injury.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- I/R:
-
Ischemia-reperfusion
- FAR:
-
Farrerol
- ROS:
-
Reactive oxygen species
- ERK:
-
Extracellular signal-regulated kinase
- CREB:
-
CAMP-response element binding protein
- MAPK:
-
Mitogen-activated protein kinase
- HR:
-
Homologous recombination
- ECA:
-
External carotid artery
- MCA:
-
Middle cerebral artery
- rCBF:
-
Regional cerebral blood flow
- tMCAO:
-
Transient middle cerebral artery occlusion
- mNSS:
-
Modified Neurological Severity Scores
- OGD/R:
-
Oxygen glucose deprivation/re-oxygenation
- BSA:
-
Bovine serum albumin
- qRT-PCR:
-
Quantitative real-time PCR
- LDH:
-
Lactate dehydrogenase
- LPS:
-
Lipopolysaccharide
- PFA:
-
Paraformaldehyde
- RT:
-
Room temperature
- PBS:
-
Phosphate-buffered solution
- PI:
-
Propidium iodide
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
- DAPI:
-
Dye 4′,6-diamidino-2-phenylindole
- MFI:
-
Mean fluorescence intensity
References
Group GNDC (2017) Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16(11):877–897. https://doi.org/10.1016/S1474-4422(17)30299-5
Collaborators GDaIIaP (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England) 388(10053):1545–1602. https://doi.org/10.1016/S0140-6736(16)31678-6
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA et al (2015) A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372(1):11–20. https://doi.org/10.1056/NEJMoa1411587
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT et al (2018) Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378(8):708–718. https://doi.org/10.1056/NEJMoa1713973
Mizuma A, You JS, Yenari MA (2018) Targeting reperfusion injury in the age of mechanical thrombectomy. Stroke 49(7):1796–1802. https://doi.org/10.1161/STROKEAHA.117.017286
Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CBLM et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet (London, England) 387(10029):1723–1731. https://doi.org/10.1016/S0140-6736(16)00163-X
Church EW, Gundersen A, Glantz MJ, Simon SD (2017) Number needed to treat for stroke thrombectomy based on a systematic review and meta-analysis. Clin Neurol Neurosurg 156:83–88. https://doi.org/10.1016/j.clineuro.2017.03.005
Jurcau A, Simion A (2021) Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: from pathophysiology to therapeutic strategies. Int J Mol Sci 23(1):14. https://doi.org/10.3390/ijms23010014
Wu L, **ong X, Wu X, Ye Y, Jian Z, Zhi Z, Gu L (2020) Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front Mol Neurosci 13:28. https://doi.org/10.3389/fnmol.2020.00028
Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M (2013) Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 47(1):9–23. https://doi.org/10.1007/s12035-012-8344-z
Zhang Z, Duan Z, Cui Y (2023) CD8+ T cells in brain injury and neurodegeneration. Front Cell Neurosci 17. https://doi.org/10.3389/fncel.2023.1281763
Lai TW, Zhang S, Wang YT (2014) Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188. https://doi.org/10.1016/j.pneurobio.2013.11.006
Zhu YM, Wang CC, Chen L, Qian LB, Ma LL, Yu J, Zhu MH, Wen CY et al (2013) Both PI3K/Akt and ERK1/2 pathways participate in the protection by dexmedetomidine against transient focal cerebral ischemia/reperfusion injury in rats. Brain Res 1494:1–8. https://doi.org/10.1016/j.brainres.2012.11.047
Endo H, Nito C, Kamada H, Nishi T, Chan PH (2006) Activation of the Akt/GSK3β signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab 26(12):1479–1489. https://doi.org/10.1038/sj.jcbfm.9600303
Noshita N, Lewén A, Sugawara T, Chan PH (2002) Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol Dis 9(3):294–304. https://doi.org/10.1006/nbdi.2002.0482
Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP (2006) Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441(7092):523–527. https://doi.org/10.1038/nature04809
Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A (1998) Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 20(4):727–740. https://doi.org/10.1016/s0896-6273(00)81011-9
Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan J-Q, Henshall DC, Simon RP (2005) CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab 25(2):234–246. https://doi.org/10.1038/sj.jcbfm.9600024
Liu R, Tang JC, Pan MX, Zhuang Y, Zhang Y, Liao HB, Zhao D, Lei Y et al (2018) ERK 1/2 activation mediates the neuroprotective effect of BpV(pic) in Focal cerebral ischemia-reperfusion injury. Neurochem Res 43(7):1424–1438. https://doi.org/10.1007/s11064-018-2558-z
Drieu A, Levard D, Vivien D, Rubio M (2018) Anti-inflammatory treatments for stroke: from bench to bedside. Ther Adv Neurol Disord 11:1756286418789854. https://doi.org/10.1177/1756286418789854
Surinkaew P, Sawaddiruk P, Apaijai N, Chattipakorn N, Chattipakorn SC (2018) Role of microglia under cardiac and cerebral ischemia/reperfusion (I/R) injury. Metab Brain Dis 33(4):1019–1030. https://doi.org/10.1007/s11011-018-0232-4
Yang C, Hawkins KE, Dore S, Candelario-Jalil E (2019) Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol 316(2):C135–C153. https://doi.org/10.1152/ajpcell.00136.2018
Rayasam A, Hsu M, Kijak JA, Kissel L, Hernandez G, Sandor M, Fabry Z (2018) Immune responses in stroke: how the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology 154(3):363–376. https://doi.org/10.1111/imm.12918
Ma N, Wei W, Fan X, Ci X (2019) Farrerol attenuates cisplatin-induced nephrotoxicity by inhibiting the reactive oxygen species-mediated oxidation, inflammation, and apoptotic signaling pathways. Front Physiol 10:1419. https://doi.org/10.3389/fphys.2019.01419
Cui B, Zhang S, Wang Y, Guo Y (2019) Farrerol attenuates beta-amyloid-induced oxidative stress and inflammation through Nrf2/Keap1 pathway in a microglia cell line. Biomed Pharmacother 109:112–119. https://doi.org/10.1016/j.biopha.2018.10.053
Yang Z, Fu Y, Liu B, Zhou E, Liu Z, Song X, Li D, Zhang N (2013) Farrerol regulates antimicrobial peptide expression and reduces Staphylococcus aureus internalization into bovine mammary epithelial cells. Microb Pathog 65:1–6. https://doi.org/10.1016/j.micpath.2013.08.002
Cui B, Guo X, You Y, Fu R (2019) Farrerol attenuates MPP(+) -induced inflammatory response by TLR4 signaling in a microglia cell line. Phytother Res 33(4):1134–1141. https://doi.org/10.1002/ptr.6307
Qin X, Hou X, Zhang M, Liang T, Zhi J, Han L, Li Q (2014) Relaxation of rat aorta by farrerol correlates with potency to reduce intracellular calcium of VSMCs. Int J Mol Sci 15(4):6641–6656. https://doi.org/10.3390/ijms15046641
Li QY, Chen L, Zhu YH, Zhang M, Wang YP, Wang MW (2011) Involvement of estrogen receptor-beta in farrerol inhibition of rat thoracic aorta vascular smooth muscle cell proliferation. Acta Pharmacol Sin 32(4):433–440. https://doi.org/10.1038/aps.2011.1
Hou X, Qin X, Li Q (2018) Structureactivity associations in novel farrerol derivatives with vasorelaxant properties. Mol Med Rep 18(5):4709–4715. https://doi.org/10.3892/mmr.2018.9439
Dai F, Gao L, Zhao Y, Wang C, **e S (2016) Farrerol inhibited angiogenesis through Akt/mTOR, Erk and Jak2/Stat3 signal pathway. Phytomedicine 23(7):686–693. https://doi.org/10.1016/j.phymed.2016.03.008
Li B, Chen P, Wang JH, Li L, Gong JL, Yao H (2019) Farrerol overcomes the invasiveness of lung squamous cell carcinoma cells by regulating the expression of inducers of epithelial mesenchymal transition. Microb Pathog 131:277. https://doi.org/10.1016/j.micpath.2018.04.052
Zhang T, Liu H, Liu M, Wang C (2021) Farrerol suppresses the progression of laryngeal squamous cell carcinoma via the mitochondria-mediated pathway. Eur J Pharmacol 913:174636. https://doi.org/10.1016/j.ejphar.2021.174636
Zhang W, Chen Y, Yang J, Zhang J, Yu J, Wang M, Zhao X, Wei K et al (2020) A high-throughput small molecule screen identifies farrerol as a potentiator of CRISPR/Cas9-mediated genome editing. Elife 9:e56008. https://doi.org/10.7554/eLife.56008
Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K et al (2019) Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 565(7738):246–250. https://doi.org/10.1038/s41586-018-0824-5
Cui Y, Zhang Y, Zhao X, Shao L, Liu G, Sun C, Xu R, Zhang Z (2021) ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun 93:312–321. https://doi.org/10.1016/j.bbi.2021.01.003
Liu F, Schafer DP, McCullough LD (2009) TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods 179(1):1–8. https://doi.org/10.1016/j.jneumeth.2008.12.028
Chen H-Z, Guo S, Li Z-Z, Lu Y, Jiang D-S, Zhang R, Lei H, Gao L et al (2014) A critical role for interferon regulatory factor 9 in cerebral ischemic stroke. J Neurosci 34(36):11897–11912. https://doi.org/10.1523/JNEUROSCI.1545-14.2014
Quaegebeur A, Segura I, Schmieder R, Verdegem D, Decimo I, Bifari F, Dresselaers T, Eelen G et al (2016) Deletion or inhibition of the oxygen sensor PHD1 protects against ischemic stroke via reprogramming of neuronal metabolism. Cell Metab 23(2):280–291. https://doi.org/10.1016/j.cmet.2015.12.007
Sasaki T, Takemori H, Yagita Y, Terasaki Y, Uebi T, Horike N, Takagi H, Susumu T et al (2011) SIK2 is a key regulator for neuronal survival after ischemia via TORC1-CREB. Neuron 69(1):106–119. https://doi.org/10.1016/j.neuron.2010.12.004
Zhang SZ, Wang QQ, Yang QQ, Gu HY, Yin YQ, Li YD, Hou JC, Chen R et al (2019) NG2 glia regulate brain innate immunity via TGF-beta2/TGFBR2 axis. BMC Med 17(1):204. https://doi.org/10.1186/s12916-019-1439-x
Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY et al (2013) Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature 494(7435):90–94. https://doi.org/10.1038/nature11748
Young K, Morrison H (2018) Quantifying microglia morphology from photomicrographs of immunohistochemistry prepared tissue using ImageJ. J Visual Exp 136:57648. https://doi.org/10.3791/57648
Wang A, Jia B, Zhang X, Huo X, Chen J, Gui L, Cai Y, Guo Z et al (2023) Efficacy and safety of butylphthalide in patients with acute ischemic stroke: a randomized clinical trial. JAMA Neurol 80(8):851–859. https://doi.org/10.1001/jamaneurol.2023.1871
Morioka T, Kalehua AN, Streit WJ (1993) Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J Comp Neurol 327(1):123–132. https://doi.org/10.1002/cne.903270110
Chen A, **ong LJ, Tong Y, Mao M (2013) The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep 1(2):167–176. https://doi.org/10.3892/br.2012.48
Chen J, Simon RP, Nagayama T, Zhu R, Loeffert JE, Watkins SC, Graham SH (2000) Suppression of endogenous bcl-2 expression by antisense treatment exacerbates ischemic neuronal death. J Cereb Blood Flow Metab 20(7):1033–1039. https://doi.org/10.1097/00004647-200007000-00002
**e F, Li BX, Kassenbrock A, Xue C, Wang X, Qian DZ, Sears RC, **ao X (2015) Identification of a potent inhibitor of CREB-mediated gene transcription with efficacious in vivo anticancer activity. J Med Chem 58(12):5075–5087. https://doi.org/10.1021/acs.jmedchem.5b00468
Li BX, Gardner R, Xue C, Qian DZ, **e F, Thomas G, Kazmierczak SC, Habecker BA et al (2016) Systemic inhibition of CREB is well-tolerated in vivo. Sci Rep 6(1):34513. https://doi.org/10.1038/srep34513
Li Y, Zeng Y, Meng T, Gao X, Huang B, He D, Ran X, Du J et al (2019) Farrerol protects dopaminergic neurons in a rat model of lipopolysaccharide-induced Parkinson’s disease by suppressing the activation of the AKT and NF-kappaB signaling pathways. Int Immunopharmacol 75:105739. https://doi.org/10.1016/j.intimp.2019.105739
** K, Mao XO, Simon RP, Greenberg DA (2001) Cyclic AMP response element binding protein (CREB) and CREB binding protein (CBP) in global cerebral ischemia. J Mol Neurosci: MN 16(1):49–56. https://doi.org/10.1385/JMN:16:1:49
Steven A, Seliger B (2016) Control of CREB expression in tumors: from molecular mechanisms and signal transduction pathways to therapeutic target. Oncotarget 7(23):35454–35465. https://doi.org/10.18632/oncotarget.7721
Wen AY, Sakamoto KM, Miller LS (2010) The role of the transcription factor CREB in immune function. J Immunol 185(11):6413–6419. https://doi.org/10.4049/jimmunol.1001829
Wu Y, Yao J, Feng K (2020) miR-124-5p/NOX2 axis modulates the ROS production and the inflammatory microenvironment to protect against the cerebral I/R injury. Neurochem Res 45(2):404–417. https://doi.org/10.1007/s11064-019-02931-0
Liou K-T, Shen Y-C, Chen C-F, Tsao C-M, Tsai S-K (2003) Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res 992(2):159–166. https://doi.org/10.1016/j.brainres.2003.08.026
Yan C, Zhang X, Miao J, Yuan H, Liu E, Liang T, Li Q (2020) Farrerol directly targets GSK-3beta to activate Nrf2-ARE pathway and protect EA.hy926 cells against oxidative stress-induced injuries. Oxid Med Cell Longev 2020:5967434. https://doi.org/10.1155/2020/5967434
CiLvWangWangPengQinCheng XHLXLFXG (2015) The antioxidative potential of farrerol occurs via the activation of Nrf2 mediated HO-1 signaling in RAW 264.7 cells. Chem Biol Interact 239:192–199. https://doi.org/10.1016/j.cbi.2015.06.032
Li Y, Gong Q, Guo W, Kan X, Xu D, Ma H, Fu S, Liu J (2018) Farrerol relieve lipopolysaccharide (LPS)-induced mastitis by inhibiting AKT/NF-kappaB p65, ERK1/2 and P38 signaling pathway. Int J Mol Sci 19(6):1770. https://doi.org/10.3390/ijms19061770
Funding
This work was supported by Science and Technology Support Plan for Youth Innovation of Colleges and Universities of Shandong Province of China (Grant number 2022KJ146, Yu Cui) and Natural Science Foundation of Shandong Province (Grant number ZR202102190696, Rui Xu). Rui Xu also received research support from the Project of Bei**g DNVA Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. R. Zhao and X. Zhou designed the research and wrote the first draft of the manuscript. Z. Zhao and W. Liu prepared the material. R. Zhao, M. Lv, Z. Zhang, and C. Wang performed the experiments. X. Zhou, T. Li, Z. Yang, and Q. Wan collected and analyzed the data. R. Xu and Y. Cui conceptualized the research and directed the study. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments were conducted in compliance with National Institutes of Health Guidelines and were approved by the Qingdao University Laboratory Animal Welfare Ethics Committee (July 23, 2021/ No. 20210820C572720220520068).
Consent to Participate
Not applicable.
Consent for Publication
All authors have approved the contents of this manuscript and provided consent for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, R., Zhou, X., Zhao, Z. et al. Farrerol Alleviates Cerebral Ischemia–Reperfusion Injury by Promoting Neuronal Survival and Reducing Neuroinflammation. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04031-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04031-9