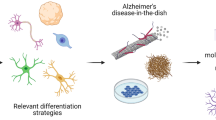
Abstract
Brain organoids, three-dimensional cell structures derived from pluripotent stem cells, closely mimic key aspects of the human brain in vitro, providing a powerful tool for studying neurodevelopment and disease. The neuroectodermal induction protocol employed for brain organoid generation primarily gives rise to the neural cellular component but lacks the vital vascular system, which is crucial for the brain functions by regulating differentiation, migration, and circuit formation, as well as delivering oxygen and nutrients. Many neurological diseases are caused by dysfunctions of cerebral microcirculation, making vascularization of human brain organoids an important tool for pathogenetic and translational research. Experimentally, the creation of vascularized brain organoids has primarily focused on the fusion of vascular and brain organoids, on organoid transplantation in vivo, and on the use of microfluidic devices to replicate the intricate microenvironment of the human brain in vitro. This review summarizes these efforts and highlights the importance of studying the neurovascular unit in a forward-looking perspective of leveraging their use for understanding and treating neurological disorders.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organoids are complex three-dimensional (3D) cellular systems derived from pluripotent stem cells (PSCs) or adult stem cells [1, 2]. Concerning the nervous system’s organoids, when the originating cells are cultured in suspension under specific conditions that reproduce embryonic development, they form structures that mimic the organ architectures and functions, including the central nervous system (CNS) as a whole (whole-brain organoids) or specific CNS areas (regional organoids) [3]. As cells develop within brain organoids, they follow a developmental timeline that is similar to in vivo neurogenesis [3]. Brain organoids are able to generate spontaneous neural activity, form functional synapses, and support interneuron migration or axonal projection, as well as interact and fuse with adjacent organoids giving rise to assembloids, mimicking the architecture and complex interactions of CNS tissues [4, 5]. Additionally, transcriptomic and epigenetic studies confirmed that brain organoids recapitulate the key molecular features of the human embryonic/fetal brain [4].
The main source of cells used for brain organoid generation is induced pluripotent stem cells (iPSCs) [6] and embryonic stem cells (ESCs) [7], both widely employed. iPSCs are derived from somatic cells primarily of the skin or of the blood, which are reprogrammed into embryonic-like states by administration of specific pluripotency transcription factors. Unlike ESCs, iPSCs do not carry the ethical concerns associated with ESCs while fully reflecting the patient’s genetic background [6, 8, 9]. Notably, iPSCs are able to differentiate in several cell types, including neuronal cells, making them particularly suitable for studying neurodegenerative disorders.
To date, disease modeling has largely relied on two-dimensional (2D) cell cultures, human biopsy specimens, and animal models. Nevertheless, the majority of human tissues are difficult to biopsy, and their use is strictly regulated, while 2D cell cultures do not fully mimic the structural organization and functions of human tissues, poorly attempting to replicate the real in vivo conditions [8]. As regards in vivo animal models, despite their ability to replicate the complexity of living organisms better than in vitro ones, their utility is limited by interspecies differences.
Recently, iPSC-derived 3D models, grown in a way that cells interact with each other and their surroundings more naturally, mimicking the in vivo conditions to a greater extent, have become key tool for disease modeling and for the development of new therapeutic strategies, closely resembling the in vivo microenvironment. In this context, iPSC-derived organoids, free floating 3D models, offer the chance to deeply investigate physiological and pathological mechanisms in a specific genetic background. Particularly, brain organoids have attracted huge interest to study neural development and as innovative tools for drug discovery and regenerative medicine [10]. The exploitation of organoid potential can be further extended to the generation of multi-unit structure called assembloid, a more sophisticated in vitro model, which attempts to recapitulate intercellular interactions among different organ-like structures [11]. Assembloids are self-organizing cellular entities emerging from the integration of distinct organoids or derived from the combination with specialized cell populations [5, 12].
So far, iPSC-derived brain organoids have been generated to model a large range of both developmental and degenerative brain disorders [13,14,15,16,17]. Nonetheless, their major limitation, as a model, is the lack of a vascular system for transporting nutrients or drugs, which in vivo occurs through microvascular cells and structures. The absence of a neurovascular system limits organoid growth, neurogenesis, and functions, restricting their potential applications [18,19,20,21,22].
Because of that, vascularization of brain organoids is one of the main sought-after advancements in the field, allowing their use for etiopathological studies and drug screening tests based on blood–brain barrier (BBB) permeability, as well as a platform for studying neurological disorders and particularly cerebrovascular diseases [14, 15, 23,24,25]. Here, we aim to review both the current state and future perspectives of vascularized human brain organoids.
Neural Organoid Generation
One of the first attempts to generate brain organoids that resemble the human brain in 3D was performed in 2013 by Lancaster and colleagues [13]. The original protocol relied on embedding PSCs in a basement membrane-like matrix to facilitate neuroepithelial development. The generation of brain organoids in this way primarily relied on intrinsic signals, thus requiring only a minimal number of growth factors and other substances for patterning, with basic fibroblast growth factor (bFGF) being used as the sole growth factor in the first 6 days. These self-develo** organoids resulted in a stochastic mixture of different brain cellular components, spanning from the retina to the hindbrain, implying low reproducibility and high variability. To overcome this aspect, subsequent protocols were developed progressively introducing inductive cues such as morphogens and signaling molecules, capable of directing the neurodevelopmental specification in a timely manner [26, 27]. Indeed, by using these protocols, 3D structures with a more specific regional identity such as the hypothalamus, midbrain, brainstem, and choroid plexus could be created [28, 29]. Organoids’ growth and maturation can continue over several months, reaching a width of several millimeters [16]. Eventually, they contain a variety of different cell populations of ectodermal origin, including multiple neuronal subtypes, astrocytes, oligodendrocytes, and outer radial glia cells [27, 30,31,32,33,34,35]. Notably, it has been described that microglia can develop within the brain organoid, simultaneously with neuroectodermal cell types, if dual SMAD inhibition is removed. Indeed, innately developed mesodermal progenitors are capable of differentiating into mature microglia under the influence of the CNS microenvironment provided by neuroectodermal cells [36].
Human brain organoids have the advantage of being able to model unique human-specific characteristics, such as the development of outer radial glial cells which largely contribute to the development of the human cerebral cortex and whose alteration can be responsible for pathological conditions [15]. Different patterned CNS organoids’ derivation protocols have been implemented over the past few years. Telencephalic aggregates were first developed to segregate GABAergic and glutamatergic neurons, while cortical glutamatergic neurons and astrocytes have been derived through the generation of cortical spheroids [26, 37]. By modifying the neurocortical induction protocol, the choroid plexus and the medial pallium-like tissues, precursors of the hippocampal telencephalic area, were established [38]. Finally, midbrain organoids containing dopaminergic neurons of the nigro-striatal pathway were generated [104, 105]. Research on rodent models has demonstrated that ECs could attract recently differentiated neurons to migrate along vessels and reach areas affected by ischemia [106]. The influence of neurovascular interactions on developmental (angiogenesis, BBB formation, mural and vascular cell development, regulation of neuronal and glial development) and reparative events in humans remains unclear since there is no relevant human model or there are models with poor human translatability. Indeed, in vitro conventional cell culture models [107], microphysiological systems [108, 109], tissue-engineered 3D models, or bioprinting [110] do not properly fulfill conditions of the human NVU microenvironment. Recreating the intricate microenvironment and the cellular interactions within the neurovascular unit becomes pivotal in comprehending its functioning and, further, in devising CNS-targeted pharmaceuticals that can effectively penetrate the brain for experimental testing. The development of vascularized organoids could overcome this obstacle. Additionally, gene expression analysis, including single-cell profiles, may reveal new players in neurovascular mechanisms.
Further, organoids could also prove extremely useful for regenerative therapies. Indeed, a differentiated human brain organoid, containing appropriate cell populations, active neural circuits, and an adequate vasculature may facilitate their use as a cell source for transplantation strategy for CNS tissue regeneration [73, 111]. Finally, vascularized human brain organoids hold the potential for application in drug screening, discovery of new potential disease biomarkers, and advancement of cutting-edge diagnostics and therapies [63, 73]. As clinical avatar, patient-specific organoids can be used for diagnostic interventions, tested with therapeutic strategies, and personalized medicine could eventually be achieved in the next future [97].
Overall, it appears clear that brain organoids, especially vascularized organoids, could prove to be a fundamental resource for understanding and treating neurological diseases.
Conclusions
Vascularized human brain organoids are still imperfect tools, and they need to be further optimized before they are able to precisely recapitulate the development, function, and pathology of the neurovascular system. Several studies demonstrated that vascularizing human brain organoids allowed for a better maturation and survival of neural cells. More specifically, better functional circuit and firing rate [61] and increased number of mature neurons [73] have been observed. Furthermore, some key aspects of the interactions between vascular cells and neural cells in health and pathology have been clarified [53, 69], including the regulation of the BBB maturation by neural cues [72].
Current research suggests that vascularized brain organoids provide better pathophysiological models compared to non-vascularized ones [61, 68]. Indeed, compared to other in vitro models, neurovascular organoids can better reproduce the cytoarchitecture of the brain, providing functional and synaptic connectivity data. Nevertheless, the generation of vascularized brain organoids requires more resources with consequent lower accessibility than non-vascularized organoids, hindering their applicability. For an extensive use of brain organoids for disease modeling and drug discovery, substantial advancements in automation and scaling up are needed.
The pathophysiology of brain diseases is influenced by blood flow, cellular composition, vascular cell-derived factors, and microglia. Recent advances in cell culture technology allowed the growth of brain organoids for long periods, paving the way to model late-onset diseases such as neurodegenerative diseases [17]. Indeed, long-term cultures of vascularized brain organoids may be very useful for modeling aging-associated diseases such as stroke or AD, while young organoids used to model fetal brain development may not accurately reproduce stroke-relevant phenotypes.
Since vascularized organoids can more easily integrate into the host tissues and may promote healing better than non-vascularized organoids, they may be an excellent source for cell transplantation in regenerative medicine approaches. Nonetheless, more studies are required to evaluate the chance to perfuse vascularized brain organoids without transplanting them in vivo. Indeed, a controlled perfusion method could assess the vessel permeability through a direct injection of blood cells into the organoid as well as reduce the characteristic necrotic core of organoids [112, 113]. Additionally, biomechanical properties of the brain tissue and its vascular system such as stiffness, viscoelasticity, and spatial organization influence physiological processes such as proliferation, migration, differentiation, and cell functions [114].
Overall, all these studies have emphasized the significance of vascularized organoids in faithfully recapitulating different aspects of the neurovascular microenvironment, paving the way for a deeper understanding of the molecular mechanisms underlying neurodegeneration in a forward-looking perspective of identifying new therapeutic strategies.
Data Availability and Code Availability
Not applicable.
References
Clevers H (2016) Modeling development and disease with organoids. Cell 165:1586–1597. https://doi.org/10.1016/j.cell.2016.05.082
Corrò C, Novellasdemunt L, Li VSW (2020) A brief history of organoids. Am J Physiol Cell Physiol 319:C151–C165. https://doi.org/10.1152/ajpcell.00120.2020
Uzquiano A, Arlotta P (2022) Brain organoids: the quest to decipher human-specific features of brain development. Curr Opin Genet Dev 75:101955. https://doi.org/10.1016/j.gde.2022.101955
Kelley KW, Pașca SP (2022) Human brain organogenesis: toward a cellular understanding of development and disease. Cell 185:42–61. https://doi.org/10.1016/j.cell.2021.10.003
Paşca SP (2019) Assembling human brain organoids. Science 363:126–127. https://doi.org/10.1126/science.aau5729
Costamagna G, Comi GP, Corti S (2021) Advancing drug discovery for neurological disorders using iPSC-derived neural organoids. Int J Mol Sci 22:2659. https://doi.org/10.3390/ijms22052659
Lee C-T, Bendriem RM, Wu WW, Shen R-F (2017) 3D brain organoids derived from pluripotent stem cells: promising experimental models for brain development and neurodegenerative disorders. J Biomed Sci 24:59. https://doi.org/10.1186/s12929-017-0362-8
Corti S, Faravelli I, Cardano M, Conti L (2015) Human pluripotent stem cells as tools for neurodegenerative and neurodevelopmental disease modeling and drug discovery. Expert Opin Drug Discov 10:615–629. https://doi.org/10.1517/17460441.2015.1037737
Faravelli I, Costamagna G, Tamanini S, Corti S (2020) Back to the origins: human brain organoids to investigate neurodegeneration. Brain Res 1727:146561. https://doi.org/10.1016/j.brainres.2019.146561
Hofer M, Lutolf MP (2021) Engineering organoids. Nat Rev Mater 6:402–420. https://doi.org/10.1038/s41578-021-00279-y
Andersen J, Revah O, Miura Y et al (2020) Generation of functional human 3D cortico-motor assembloids. Cell 183:1913-1929.e26. https://doi.org/10.1016/j.cell.2020.11.017
Pașca SP, Arlotta P, Bateup HS et al (2022) A nomenclature consensus for nervous system organoids and assembloids. Nature 609:907–910. https://doi.org/10.1038/s41586-022-05219-6
Lancaster MA, Renner M, Martin C-A et al (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379. https://doi.org/10.1038/nature12517
Kelava I, Lancaster MA (2016) Stem cell models of human brain development. Cell Stem Cell 18:736–748. https://doi.org/10.1016/j.stem.2016.05.022
Di Lullo E, Kriegstein AR (2017) The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 18:573–584. https://doi.org/10.1038/nrn.2017.107
Amin ND, Paşca SP (2018) Building models of brain disorders with three-dimensional organoids. Neuron 100:389–405. https://doi.org/10.1016/j.neuron.2018.10.007
Lancaster MA, Knoblich JA (2014) Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9:2329–2340. https://doi.org/10.1038/nprot.2014.158
Delgado AC, Ferrón SR, Vicente D et al (2014) Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 83:572–585. https://doi.org/10.1016/j.neuron.2014.06.015
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163:1064–1078. https://doi.org/10.1016/j.cell.2015.10.067
Tata M, Wall I, Joyce A et al (2016) Regulation of embryonic neurogenesis by germinal zone vasculature. Proc Natl Acad Sci U S A 113:13414–13419. https://doi.org/10.1073/pnas.1613113113
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12:723–738. https://doi.org/10.1038/nrn3114
Lancaster MA (2018) Brain organoids get vascularized. Nat Biotechnol 36:407–408. https://doi.org/10.1038/nbt.4133
Giandomenico SL, Lancaster MA (2017) Probing human brain evolution and development in organoids. Curr Opin Cell Biol 44:36–43. https://doi.org/10.1016/j.ceb.2017.01.001
Mansour AA, Schafer ST, Gage FH (2021) Cellular complexity in brain organoids: current progress and unsolved issues. Semin Cell Dev Biol 111:32–39. https://doi.org/10.1016/j.semcdb.2020.05.013
Li M, Gao L, Zhao L et al (2023) Toward the next generation of vascularized human neural organoids. Med Res Rev 43:31–54. https://doi.org/10.1002/med.21922
Paşca AM, Sloan SA, Clarke LE et al (2015) Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 12:671–678. https://doi.org/10.1038/nmeth.3415
Kadoshima T, Sakaguchi H, Nakano T et al (2013) Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell–derived neocortex. Proc Natl Acad Sci 110:20284–20289. https://doi.org/10.1073/pnas.1315710110
Susaimanickam PJ, Kiral FR, Park I-H (2022) Region specific brain organoids to study neurodevelopmental disorders. Int J Stem Cells 15:26–40. https://doi.org/10.15283/ijsc22006
Eura N, Matsui TK, Luginbühl J et al (2020) Brainstem organoids from human pluripotent stem cells. Front Neurosci 14:538. https://doi.org/10.3389/fnins.2020.00538
Sloan SA, Darmanis S, Huber N et al (2017) Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95:779-790.e6. https://doi.org/10.1016/j.neuron.2017.07.035
Pollen AA, Bhaduri A, Andrews MG et al (2019) Establishing cerebral organoids as models of human-specific brain evolution. Cell 176:743-756.e17. https://doi.org/10.1016/j.cell.2019.01.017
Camp JG, Badsha F, Florio M et al (2015) Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A 112:15672–15677. https://doi.org/10.1073/pnas.1520760112
Qian X, Nguyen HN, Song MM et al (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–1254. https://doi.org/10.1016/j.cell.2016.04.032
Birey F, Andersen J, Makinson CD et al (2017) Assembly of functionally integrated human forebrain spheroids. Nature 545:54–59. https://doi.org/10.1038/nature22330
Quadrato G, Nguyen T, Macosko EZ et al (2017) Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545:48–53. https://doi.org/10.1038/nature22047
Ormel PR, Vieira de Sá R, van Bodegraven EJ et al (2018) Microglia innately develop within cerebral organoids. Nat Commun 9:4167. https://doi.org/10.1038/s41467-018-06684-2
Mariani J, Coppola G, Zhang P et al (2015) FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162:375–390. https://doi.org/10.1016/j.cell.2015.06.034
Watanabe M, Buth JE, Haney JR et al (2022) TGFβ superfamily signaling regulates the state of human stem cell pluripotency and capacity to create well-structured telencephalic organoids. Stem Cell Reports 17:2220–2238. https://doi.org/10.1016/j.stemcr.2022.08.013
Jo J, **ao Y, Sun AX et al (2016) Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19:248–257. https://doi.org/10.1016/j.stem.2016.07.005
Smits LM, Reinhardt L, Reinhardt P et al (2019) Modeling Parkinson’s disease in midbrain-like organoids. NPJ Parkinsons Dis 5:5. https://doi.org/10.1038/s41531-019-0078-4
Galet B, Cheval H, Ravassard P (2020) Patient-derived midbrain organoids to explore the molecular basis of Parkinson’s disease. Front Neurol 11:1005. https://doi.org/10.3389/fneur.2020.01005
Licata JP, Schwab KH, Har-el Y et al (2023) Bioreactor technologies for enhanced organoid culture. Int J Mol Sci 24:11427. https://doi.org/10.3390/ijms241411427
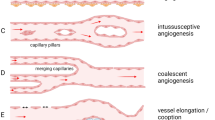
Raybaud C (2010) Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg Clin N Am 21:399–426. https://doi.org/10.1016/j.nec.2010.03.011
Risau W (1997) Mechanisms of angiogenesis. Nature 386:671–674. https://doi.org/10.1038/386671a0
Zadeh G, Guha A (2003) Angiogenesis in nervous system disorders. Neurosurgery 53:1362–1374; discussion 1374–1376. https://doi.org/10.1227/01.neu.0000093425.98136.31
Lee HS, Han J, Bai H-J, Kim K-W (2009) Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J 276:4622–4635. https://doi.org/10.1111/j.1742-4658.2009.07174.x
Iadecola C (2004) Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5:347–360. https://doi.org/10.1038/nrn1387
Schaeffer S, Iadecola C (2021) Revisiting the neurovascular unit. Nat Neurosci 24:1198–1209. https://doi.org/10.1038/s41593-021-00904-7
Iadecola C (2017) The Neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96:17–42. https://doi.org/10.1016/j.neuron.2017.07.030
Parkes I, Chintawar S, Cader MZ (2018) Neurovascular dysfunction in dementia – human cellular models and molecular mechanisms. Clin Sci 132:399–418. https://doi.org/10.1042/CS20160720
Harding A, Cortez-Toledo E, Magner NL et al (2017) Highly efficient differentiation of endothelial cells from pluripotent stem cells requires the MAPK and the PI3K pathways. Stem Cells 35:909–919. https://doi.org/10.1002/stem.2577
Cleaver O, Melton DA (2003) Endothelial signaling during development. Nat Med 9:661–668. https://doi.org/10.1038/nm0603-661
Cakir B, **ang Y, Tanaka Y et al (2019) Engineering of human brain organoids with a functional vascular-like system. Nat Methods 16:1169–1175. https://doi.org/10.1038/s41592-019-0586-5
Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV (2016) Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta 1862:887–900. https://doi.org/10.1016/j.bbadis.2015.12.016
Jolivalt CG, Lee CA, Beiswenger KK et al (2008) Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer’s disease and correction by insulin. J Neurosci Res 86:3265–3274. https://doi.org/10.1002/jnr.21787
Verdile G, Keane KN, Cruzat VF et al (2015) Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators Inflamm 2015:105828. https://doi.org/10.1155/2015/105828
Liang X, Yao Y, Lin Y et al (2019) Panaxadiol inhibits synaptic dysfunction in Alzheimer’s disease and targets the Fyn protein in APP/PS1 mice and APP-SH-SY5Y cells. Life Sci 221:35–46. https://doi.org/10.1016/j.lfs.2019.02.012
Eisenmenger LB, Peret A, Famakin BM et al (2022) Vascular contributions to Alzheimer’s disease. Transl Res S1931–5244(22):00282–00291. https://doi.org/10.1016/j.trsl.2022.12.003
Salmon I, Grebenyuk S, Fattah ARA et al (2022) Engineering neurovascular organoids with 3D printed microfluidic chips. Lab Chip 22:1615–1629. https://doi.org/10.1039/D1LC00535A
Stern CD (2005) Neural induction: old problem, new findings, yet more questions. Development 132:2007–2021. https://doi.org/10.1242/dev.01794
Shi Y, Sun L, Wang M et al (2020) Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol 18:e3000705. https://doi.org/10.1371/journal.pbio.3000705
Wörsdörfer P, Dalda N, Kern A et al (2019) Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci Rep 9:15663. https://doi.org/10.1038/s41598-019-52204-7
Pham MT, Pollock KM, Rose MD et al (2018) Generation of human vascularized brain organoids. NeuroReport 29:588–593. https://doi.org/10.1097/WNR.0000000000001014
Urich E, Patsch C, Aigner S et al (2013) Multicellular self-assembled spheroidal model of the blood brain barrier. Sci Rep 3:1500. https://doi.org/10.1038/srep01500
Cho C-F, Wolfe JM, Fadzen CM et al (2017) Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat Commun 8:15623. https://doi.org/10.1038/ncomms15623
Nzou G, Wicks RT, Wicks EE et al (2018) Human cortex spheroid with a functional blood brain barrier for high-throughput neurotoxicity screening and disease modeling. Sci Rep 8:7413. https://doi.org/10.1038/s41598-018-25603-5
Nzou G, Wicks RT, VanOstrand NR et al (2020) Multicellular 3D neurovascular unit model for assessing hypoxia and neuroinflammation induced blood-brain barrier dysfunction. Sci Rep 10:9766. https://doi.org/10.1038/s41598-020-66487-8
Song L, Yuan X, Jones Z et al (2019) Assembly of human stem cell-derived cortical spheroids and vascular spheroids to model 3-D brain-like tissues. Sci Rep 9:5977. https://doi.org/10.1038/s41598-019-42439-9
Kook MG, Lee S-E, Shin N et al (2022) Generation of cortical brain organoid with vascularization by assembling with vascular spheroid. Int J Stem Cells 15:85–94. https://doi.org/10.15283/ijsc21157
Ham O, ** YB, Kim J, Lee M-O (2020) Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem Biophys Res Commun 521:84–90. https://doi.org/10.1016/j.bbrc.2019.10.079
Ahn Y, An J-H, Yang H-J et al (2021) Human blood vessel organoids penetrate human cerebral organoids and form a vessel-like system. Cells 10:2036. https://doi.org/10.3390/cells10082036
Sun X-Y, Ju X-C, Li Y et al (2022) Generation of vascularized brain organoids to study neurovascular interactions. eLife 11:e76707. https://doi.org/10.7554/eLife.76707
Mansour AA, Gonçalves JT, Bloyd CW et al (2018) An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 36:432–441. https://doi.org/10.1038/nbt.4127
Yu J (2021) Vascularized organoids: a more complete model. Int J Stem Cells 14:127–137. https://doi.org/10.15283/ijsc20143
Matsui TK, Tsuru Y, Hasegawa K, Kuwako K-I (2021) Vascularization of human brain organoids. Stem Cells 39:1017–1024. https://doi.org/10.1002/stem.3368
Wilson MN, Thunemann M, Liu X et al (2022) Multimodal monitoring of human cortical organoids implanted in mice reveal functional connection with visual cortex. Nat Commun 13:7945. https://doi.org/10.1038/s41467-022-35536-3
Chen EP, Toksoy Z, Davis BA, Geibel JP (2021) 3D bioprinting of vascularized tissues for in vitro and in vivo applications. Front Bioeng Biotechnol 9:664188. https://doi.org/10.3389/fbioe.2021.664188
Song JW, Munn LL (2011) Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A 108:15342–15347. https://doi.org/10.1073/pnas.1105316108
Bischel LL, Young EWK, Mader BR, Beebe DJ (2013) Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 34:1471–1477. https://doi.org/10.1016/j.biomaterials.2012.11.005
Miller RG, Mitchell JD, Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev CD001447. https://doi.org/10.1002/14651858.CD001447.pub3
Nashimoto Y, Hayashi T, Kunita I et al (2017) Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr Biol 9:506–518. https://doi.org/10.1039/c7ib00024c. (Camb)
Wang X, Phan DTT, Sobrino A et al (2016) Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 16:282–290. https://doi.org/10.1039/c5lc01050k
Bogorad MI, DeStefano J, Karlsson J et al (2015) Review: in vitro microvessel models. Lab Chip 15:4242–4255. https://doi.org/10.1039/c5lc00832h
Haase K, Kamm RD (2017) Advances in on-chip vascularization. Regen Med 12:285–302. https://doi.org/10.2217/rme-2016-0152
Grebenyuk S, Ranga A (2019) Engineering organoid vascularization. Front Bioeng Biotechnol 7:39. https://doi.org/10.3389/fbioe.2019.00039
Shirure VS, Hughes CCW, George SC (2021) Engineering vascularized organoid-on-a-chip models. Annu Rev Biomed Eng 23:141–167. https://doi.org/10.1146/annurev-bioeng-090120-094330
Park SE, Georgescu A, Huh D (2019) Organoids-on-a-chip. Science 364:960–965. https://doi.org/10.1126/science.aaw7894
Mandrycky CJ, Howard CC, Rayner SG et al (2021) Organ-on-a-chip systems for vascular biology. J Mol Cell Cardiol 159:1–13. https://doi.org/10.1016/j.yjmcc.2021.06.002
Song J, Bang S, Choi N, Kim HN (2022) Brain organoid-on-a-chip: a next-generation human brain avatar for recapitulating human brain physiology and pathology. Biomicrofluidics 16:061301. https://doi.org/10.1063/5.0121476
Homan KA, Gupta N, Kroll KT et al (2019) Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16:255–262. https://doi.org/10.1038/s41592-019-0325-y
Maoz BM, Herland A, FitzGerald EA et al (2018) A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat Biotechnol 36:865–874. https://doi.org/10.1038/nbt.4226
Phan DT, Bender RHF, Andrejecsk JW et al (2017) Blood-brain barrier-on-a-chip: microphysiological systems that capture the complexity of the blood-central nervous system interface. Exp Biol Med 242:1669–1678. https://doi.org/10.1177/1535370217694100. (Maywood)
Engelhardt B, Sorokin L (2009) The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31:497–511. https://doi.org/10.1007/s00281-009-0177-0
Abbott NJ, Patabendige AAK, Dolman DEM et al (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Miller JA, Ding S-L, Sunkin SM et al (2014) Transcriptional landscape of the prenatal human brain. Nature 508:199–206. https://doi.org/10.1038/nature13185
Song HW, Foreman KL, Gastfriend BD et al (2020) Transcriptomic comparison of human and mouse brain microvessels. Sci Rep 10:12358. https://doi.org/10.1038/s41598-020-69096-7
Zheng F, **ao Y, Liu H et al (2021) Patient-specific organoid and organ-on-a-chip: 3D cell-culture meets 3D printing and numerical simulation. Adv Biol 5:e2000024. https://doi.org/10.1002/adbi.202000024. (Weinh)
Yang S, Zhang J, Chen L (2020) The cells involved in the pathological process of diabetic retinopathy. Biomed Pharmacother 132:110818. https://doi.org/10.1016/j.biopha.2020.110818
Verbeek MM, de Waal RM, Schipper JJ, Van Nostrand WE (1997) Rapid degeneration of cultured human brain pericytes by amyloid beta protein. J Neurochem 68:1135–1141. https://doi.org/10.1046/j.1471-4159.1997.68031135.x
Iadecola C (2013) The pathobiology of vascular dementia. Neuron 80:844–866. https://doi.org/10.1016/j.neuron.2013.10.008
Andjelkovic AV, Stamatovic SM, Phillips CM et al (2020) Modeling blood-brain barrier pathology in cerebrovascular disease in vitro: current and future paradigms. Fluids Barriers CNS 17:44. https://doi.org/10.1186/s12987-020-00202-7
Ding Y, Shusta EV, Palecek SP (2021) Integrating in vitro disease models of the neurovascular unit into discovery and development of neurotherapeutics. Curr Opin Biomed Eng 20:100341. https://doi.org/10.1016/j.cobme.2021.100341
Pérez-López A, Torres-Suárez AI, Martín-Sabroso C, Aparicio-Blanco J (2023) An overview of in vitro 3D models of the blood-brain barrier as a tool to predict the in vivo permeability of nanomedicines. Adv Drug Deliv Rev 196:114816. https://doi.org/10.1016/j.addr.2023.114816
De Luca C, Colangelo AM, Virtuoso A et al (2020) Neurons, glia, extracellular matrix and neurovascular unit: a systems biology approach to the complexity of synaptic plasticity in health and disease. Int J Mol Sci 21:1539. https://doi.org/10.3390/ijms21041539
Elorza Ridaura I, Sorrentino S, Moroni L (2021) Parallels between the develo** vascular and neural systems: signaling pathways and future perspectives for regenerative medicine. Adv Sci 8:e2101837. https://doi.org/10.1002/advs.202101837. (Weinh)
Kojima T, Hirota Y, Ema M et al (2010) Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 28:545–554. https://doi.org/10.1002/stem.306
Eigenmann DE, Xue G, Kim KS et al (2013) Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 10:33. https://doi.org/10.1186/2045-8118-10-33
Park T-E, Mustafaoglu N, Herland A et al (2019) Hypoxia-enhanced blood-brain barrier chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat Commun 10:2621. https://doi.org/10.1038/s41467-019-10588-0
Vatine GD, Barrile R, Workman MJ et al (2019) Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 24:995-1005.e6. https://doi.org/10.1016/j.stem.2019.05.011
Zhao X, Xu Z, **ao L et al (2021) Review on the vascularization of organoids and organoids-on-a-chip. Front Bioeng Biotechnol 9:637048. https://doi.org/10.3389/fbioe.2021.637048
Eichmüller OL, Knoblich JA (2022) Human cerebral organoids - a new tool for clinical neurology research. Nat Rev Neurol 18:661–680. https://doi.org/10.1038/s41582-022-00723-9
Zenaro E, Pietronigro E, Della Bianca V et al (2015) Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 21:880–886. https://doi.org/10.1038/nm.3913
Baufeld C, O’Loughlin E, Calcagno N et al (2018) Differential contribution of microglia and monocytes in neurodegenerative diseases. J Neural Transm 125:809–826. https://doi.org/10.1007/s00702-017-1795-7. (Vienna)
Castillo Ransanz L, Van Altena PFJ, Heine VM, Accardo A (2022) Engineered cell culture microenvironments for mechanobiology studies of brain neural cells. Front Bioeng Biotechnol 10:1096054. https://doi.org/10.3389/fbioe.2022.1096054
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. This work was partially supported by the Italian Ministry of Health (Ministero della Salute), and Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico Grant Ricerca Corrente 2022 to GPC, by the Italian Ministry of Education and Research (MUR), "Dipartimenti di Eccellenza Program 2023–2027 -Dept. of Pathophysiology and Transplantation, University of Milan” and by the Italian Ministry of Health “Hub Life Science- Diagnostica Avanzata (HLS-DA), PNC-E3-2022-23683266– CUP: C43C22001630001”. We would like to thank “Associazione Centro Dino Ferrari” for its support. Figures were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
EA, SC, MR, VM, LB, LQ, and LS drafted the manuscript for intellectual content and collected and analyzed the data. LB and LQ prepared the figures. SC, GPC, MR, VM, LO, MM, NT, FV, AR, and EA revised the manuscript for intellectual content. All the authors read, contributed to the article, and approved the submitted version.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rizzuti, M., Melzi, V., Brambilla, L. et al. Sha** the Neurovascular Unit Exploiting Human Brain Organoids. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03998-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03998-9