Abstract
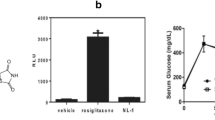
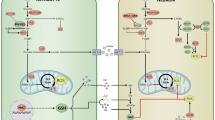
Mitochondrial dysfunction and oxidative stress play a key role in ischemia/reperfusion (I/R) induced brain injury. We previously showed that ubiquilin-1 (Ubqln1), a ubiquitin-like protein, improves proteostasis and protects brains against oxidative stress and I/R induced brain injury. We demonstrate here that nialamide (NM), a non-selective monoamine oxidase (MAO) inhibitor, upregulated Ublqn1 and protected neurons from oxygen-glucose deprivation- and I/R-caused cell death in in vitro and in vivo, respectively. Post-ischemic administration of the NM in a stroke mouse model even at 3 h following I/R still reduced neuronal injury and improved functional recovery and survival. Treating stroke animals with NM also increased the association of Ubqln1 with mitochondria and decreased the total oxidized and polyubiquitinated protein levels. Intriguingly, NM-enhanced proteostasis was also associated with reduced I/R-caused neuroinflammation, as reflected by attenuated activation of microglia and astrocytes as well as reduced TNF-α level. Thus, our results suggest that MAO inhibition-induced neuroprotection following I/R involves improved proteostasis and reduced neuroinflammation.







Similar content being viewed by others
References
Katan M, Luft A (2018) Global burden of stroke. Semin Neurol 38(2):208–211. https://doi.org/10.1055/s-0038-1649503
Cao W, Ling Y, Wu F, Yang L, Cheng X, Dong Q (2015) Higher fasting glucose next day after intravenous thrombolysis is independently associated with poor outcome in acute ischemic stroke. J Stroke Cerebrovasc Dis 24(1):100–103. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.07.029
Li J, Ma X, Yu W, Lou Z, Mu D, Wang Y, Shen B, Qi S (2012) Reperfusion promotes mitochondrial dysfunction following focal cerebral ischemia in rats. PLoS One 7(9):e46498. https://doi.org/10.1371/journal.pone.0046498
** R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc Biol 87(5):779–789. https://doi.org/10.1189/jlb.1109766
Shih JC, Chen K (2004) Regulation of MAO-A and MAO-B gene expression. Curr Med Chem 11(15):1995–2005
Wang CC, Borchert A, Ugun-Klusek A, Tang LY, Lui WT, Chu CY, Billett E, Kuhn H et al (2011) Monoamine oxidase a expression is vital for embryonic brain development by modulating developmental apoptosis. J Biol Chem 286(32):28322–28330. https://doi.org/10.1074/jbc.M111.241422
Tong J, Meyer JH, Furukawa Y, Boileau I, Chang LJ, Wilson AA, Houle S, Kish SJ (2013) Distribution of monoamine oxidase proteins in human brain: Implications for brain imaging studies. J Cereb Blood Flow Metab 33(6):863–871. https://doi.org/10.1038/jcbfm.2013.19
Kaludercic N, Carpi A, Menabo R, Di Lisa F, Paolocci N (2011) Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta 1813(7):1323–1332. https://doi.org/10.1016/j.bbamcr.2010.09.010
Deshwal S, Di Sante M, Di Lisa F, Kaludercic N (2017) Emerging role of monoamine oxidase as a therapeutic target for cardiovascular disease. Curr Opin Pharmacol 33:64–69. https://doi.org/10.1016/j.coph.2017.04.003
Ko HS, Uehara T, Tsuruma K, Nomura Y (2004) Ubiquilin interacts with ubiquitylated proteins and proteasome through its ubiquitin-associated and ubiquitin-like domains. FEBS Lett 566(1–3):110–114
Wang H, Monteiro MJ (2007) Ubiquilin interacts and enhances the degradation of expanded-polyglutamine proteins. Biochem Biophys Res Commun 360(2):423–427. https://doi.org/10.1016/j.bbrc.2007.06.097
Liu Y, Qiao F, Wang H (2017) Enhanced Proteostasis in post-ischemic stroke mouse brains by Ubiquilin-1 promotes functional recovery. Cell Mol Neurobiol 37(7):1325–1329. https://doi.org/10.1007/s10571-016-0451-3
Hu C, Tian Y, Xu H, Pan B, Terpstra EM, Wu P, Wang H, Li F et al (2018) Inadequate ubiquitination-proteasome coupling contributes to myocardial ischemia-reperfusion injury. J Clin Invest 128(12):5294–5306. https://doi.org/10.1172/JCI98287
Liu Y, Lu L, Hettinger CL, Dong G, Zhang D, Rezvani K, Wang X, Wang H (2014) Ubiquilin-1 protects cells from oxidative stress and ischemic stroke caused tissue injury in mice. J Neurosci 34(8):2813–2821. https://doi.org/10.1523/JNEUROSCI.3541-13.2014
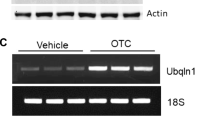
Liu Y, Min JW, Feng S, Subedi K, Qiao F, Mammenga E, Callegari E, Wang H (2019) Therapeutic role of a cysteine precursor, OTC, in ischemic stroke is mediated by improved Proteostasis in mice. Transl Stroke Res. https://doi.org/10.1007/s12975-019-00707-w
Dong G, Callegari EA, Gloeckner CJ, Ueffing M, Wang H (2012) Prothymosin-alpha interacts with mutant huntingtin and suppresses its cytotoxicity in cell culture. J Biol Chem 287(2):1279–1289. https://doi.org/10.1074/jbc.M111.294280
Dong G, Gross K, Qiao F, Ferguson J, Callegari EA, Rezvani K, Zhang D, Gloeckner CJ et al (2012) Calretinin interacts with huntingtin and reduces mutant huntingtin-caused cytotoxicity. J Neurochem 123(3):437–446. https://doi.org/10.1111/j.1471-4159.2012.07919.x
Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M et al (2005) Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab 25(2):281–290. https://doi.org/10.1038/sj.jcbfm.9600034
Min JW, Lu L, Freeling JL, Martin DS, Wang H (2016) USP14 inhibitor attenuates cerebral ischemia/reperfusion-induced neuronal injury in mice. J Neurochem 140:826–833. https://doi.org/10.1111/jnc.13941
Min JW, Liu Y, Wang D, Qiao F, Wang H (2018) The non-peptidic delta-opioid receptor agonist Tan-67 mediates neuroprotection post-ischemically and is associated with altered amyloid precursor protein expression, maturation and processing in mice. J Neurochem 144(3):336–347. https://doi.org/10.1111/jnc.14265
Liu Y, Qiao F, Wang H (2016) Enhanced Proteostasis in post-ischemic stroke mouse brains by Ubiquilin-1 promotes functional recovery. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-016-0451-3
Chen JL, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M (2001) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32(11):2682–2688. https://doi.org/10.1161/hs1101.098367
Grice GL, Nathan JA (2016) The recognition of ubiquitinated proteins by the proteasome. Cell Mol Life Sci 73(18):3497–3506. https://doi.org/10.1007/s00018-016-2255-5
Speiser Z, Mayk A, Litinetsky L, Fine T, Nyska A, Blaugrund E, Cohen S (2007) Rasagiline is neuroprotective in an experimental model of brain ischemia in the rat. J Neural Transm (Vienna) 114(5):595–605. https://doi.org/10.1007/s00702-006-0612-5
Speiser Z, Mayk A, Eliash S, Cohen S (1999) Studies with rasagiline, a MAO-B inhibitor, in experimental focal ischemia in the rat. J Neural Transm (Vienna) 106(7–8):593–606. https://doi.org/10.1007/s007020050182
Kato M, Iwata H, Okamoto M, Ishii T, Narita H (2000) Focal cerebral ischemia-induced escape deficit in rats is ameliorated by a reversible inhibitor of monoamine oxidase-a: Implications for a novel animal model of post-stroke depression. Biol Pharm Bull 23(4):406–410
Uzdensky A, Demyanenko S, Fedorenko G, Lapteva T, Fedorenko A (2017) Protein profile and morphological alterations in penumbra after focal Photothrombotic infarction in the rat cerebral cortex. Mol Neurobiol 54(6):4172–4188. https://doi.org/10.1007/s12035-016-9964-5
Gaudet R, Welch KM, Chabi E, Wang TP (1978) Effect of transient ischemia on monoamine levels in the cerebral cortex of gerbils. J Neurochem 30(4):751–757
Cvejic V, Micic DV, Djuricic BM, Mrsulja BJ, Mrsulja BB (1980) Monoamines and related enzymes in cerebral cortex and basal ganglia following transient ischemia in gerbils. Acta Neuropathol 51(1):71–77
Itakura E, Zavodszky E, Shao S, Wohlever ML, Keenan RJ, Hegde RS (2016) Ubiquilins chaperone and triage mitochondrial membrane proteins for degradation. Mol Cell 63(1):21–33. https://doi.org/10.1016/j.molcel.2016.05.020
Whiteley AM, Prado MA, Peng I, Abbas AR, Haley B, Paulo JA, Reichelt M, Katakam A et al (2017) Ubiquilin1 promotes antigen-receptor mediated proliferation by eliminating mislocalized mitochondrial proteins. Elife 6. https://doi.org/10.7554/eLife.26435
Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA (2019) Neuroinflammation: Friend and foe for ischemic stroke. J Neuroinflammation 16(1):142. https://doi.org/10.1186/s12974-019-1516-2
Bragoszewski P, Turek M, Chacinska A (2017) Control of mitochondrial biogenesis and function by the ubiquitin-proteasome system. Open Biol 7(4):170007. https://doi.org/10.1098/rsob.170007
Qiao F, Longley KR, Feng S, Schnack S, Gao H, Li Y, Schlenker EH, Wang H (2017) Reduced body weight gain in ubiquilin-1 transgenic mice is associated with increased expression of energy-sensing proteins. Physiol Rep 5(8):e13260. https://doi.org/10.14814/phy2.13260
Ran M, Li Z, Yang L, Tong L, Zhang L, Dong H (2015) Calorie restriction attenuates cerebral ischemic injury via increasing SIRT1 synthesis in the rat. Brain Res 1610:61–68. https://doi.org/10.1016/j.brainres.2015.03.043
Guo JM, Shu H, Wang L, Xu JJ, Niu XC, Zhang L (2017) SIRT1-dependent AMPK pathway in the protection of estrogen against ischemic brain injury. CNS Neurosci Ther 23(4):360–369. https://doi.org/10.1111/cns.12686
Adegoke OO, Qiao F, Liu Y, Longley K, Feng S, Wang H (2017) Overexpression of Ubiquilin-1 alleviates Alzheimer's disease-caused cognitive and motor deficits and reduces amyloid-beta accumulation in mice. J Alzheimers Dis 59(2):575–590. https://doi.org/10.3233/JAD-170173
Hiltunen M, Makinen P, Peraniemi S, Sivenius J, van Groen T, Soininen H, Jolkkonen J (2009) Focal cerebral ischemia in rats alters APP processing and expression of Abeta peptide degrading enzymes in the thalamus. Neurobiol Dis 35(1):103–113. https://doi.org/10.1016/j.nbd.2009.04.009
Krishnan KR (2007) Revisiting monoamine oxidase inhibitors. J Clin Psychiatry 68(Suppl 8):35–41
Wimbiscus M, Kostenko O, Malone D (2010) MAO inhibitors: Risks, benefits, and lore. Cleve Clin J Med 77(12):859–882. https://doi.org/10.3949/ccjm.77a.09103
Entzeroth MR, Ratty AK (2017) Monoamine Oxidase Inhibitors—Revisiting a Therapeutic Principle. Open J Depress 6:31–68
Funding
This study was funded by the National Institute of Neurological Disorders and Stroke under research grant NS088084.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed in the study. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Feng, S., Subedi, K. et al. Attenuation of Ischemic Stroke-Caused Brain Injury by a Monoamine Oxidase Inhibitor Involves Improved Proteostasis and Reduced Neuroinflammation. Mol Neurobiol 57, 937–948 (2020). https://doi.org/10.1007/s12035-019-01788-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01788-2