Abstract
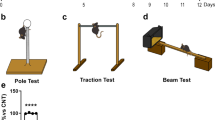
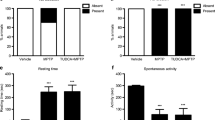
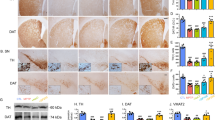
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by non-motor and motor disabilities. This study investigated whether succinobucol (SUC) could mitigate nigrostriatal injury caused by intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in mice. Moreover, the effects of SUC against MPTP-induced behavioral impairments and neurochemical changes were also evaluated. The quantification of tyrosine hydroxylase-positive (TH+) cells was also performed in primary mesencephalic cultures to evaluate the effects of SUC against 1-methyl-4-phenylpyridinium (MPP+) toxicity in vitro. C57BL/6 mice were treated with SUC (10 mg/kg/day, intragastric (i.g.)) for 30 days, and thereafter, animals received MPTP infusion (1 mg/nostril) and SUC treatment continued for additional 15 days. MPTP-infused animals displayed significant non-motor symptoms including olfactory and short-term memory deficits evaluated in the olfactory discrimination, social recognition, and water maze tasks. These behavioral impairments were accompanied by inhibition of mitochondrial NADH dehydrogenase activity (complex I), as well as significant decrease of TH and dopamine transporter (DAT) immunoreactivity in the substantia nigra pars compacta and striatum. Although SUC treatment did not rescue NADH dehydrogenase activity inhibition, it was able to blunt MPTP-induced behavioral impairments and prevented the decrease in TH and DAT immunoreactivities in substantia nigra (SN) and striatum. SUC also suppressed striatal astroglial activation and increased interleukin-6 levels in MPTP-intoxicated mice. Furthermore, SUC significantly prevented the loss of TH+ neurons induced by MPP+ in primary mesencephalic cultures. These results provide new evidence that SUC treatment counteracts early non-motor symptoms and neurodegeneration/neuroinflammation in the nigrostriatal pathway induced by intranasal MPTP administration in mice by modulating events downstream to the mitochondrial NADH dehydrogenase inhibition.







Similar content being viewed by others
References
Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302:819–22. doi:10.1126/science.1087753
Hirsch EC, Jenner P, Przedborski S (2013) Pathogenesis of Parkinson’s disease. Mov Disord 28:24–30. doi:10.1002/mds.25032
Klockgether T (2004) Parkinson’s disease: clinical aspects. Cell Tissue Res 318:115–20. doi:10.1007/s00441-004-0975-6
De Jesus-Cortes H, Xu P, Drawbridge J, Estill SJ, Huntington P, Tran S, Britt J, Tesla R, Morlock L, Naidoo J, Melito LM, Wang G, Williams NS, Ready JM, McKnight SL, Pieper AA (2012) Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc Natl Acad Sci U S A 109:17010–5. doi:10.1073/pnas.1213956109
Aarsland D, Taylor JP, Weintraub D (2014) Psychiatric issues in cognitive impairment. Mov Disord 29:651–62. doi:10.1002/mds.25873
Lima MM, Martins EF, Delattre AM, Proenca MB, Mori MA, Carabelli B, Ferraz AC (2012) Motor and non-motor features of Parkinson’s disease—a review of clinical and experimental studies. CNS Neurol Disord Drug Targets 11:439–49
Prediger RD, Aguiar AS Jr, Rojas-Mayorquin AE, Figueiredo CP, Matheus FC, Ginestet L, Chevarin C, Bel ED, Mongeau R, Hamon M, Lanfumey L, Raisman-Vozari R (2010) Single intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice models early preclinical phase of Parkinson’s disease. Neurotox Res 17:114–29. doi:10.1007/s12640-009-9087-0
Committee GPsdSS (2002) Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov Disord 17:60–7
Emre M (2003) Dementia associated with Parkinson’s disease. Lancet Neurol 2:229–37
Dubois B, Pillon B (1997) Cognitive deficits in Parkinson’s disease. J Neurol 244:2–8
Tolosa E, Compta Y, Gaig C (2007) The premotor phase of Parkinson’s disease. Parkinsonism Relat Disord 13(Suppl):S2–7. doi:10.1016/j.parkreldis.2007.06.007
Abou-Sleiman PM, Muqit MM, Wood NW (2006) Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci 7:207–19. doi:10.1038/nrn1868
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Gao HM, Liu B, Zhang W, Hong JS (2003) Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci 24:395–401. doi:10.1016/S0165-6147(03)00176-7
Nagatsu T, Mogi M, Ichinose H and Togari A (2000) Cytokines in Parkinson’s disease. J Neural Transm Suppl:143-51.
Nagatsu T, Sawada M (2005) Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Des 11:999–1016
Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5:1403–9. doi:10.1038/70978
Wang WF, Wu SL, Liou YM, Wang AL, Pawlak CR, Ho YJ (2009) MPTP lesion causes neuroinflammation and deficits in object recognition in Wistar rats. Behav Neurosci 123:1261–70. doi:10.1037/a0017401
Franco-Iborra S, Vila M and Perier C (2015) The Parkinson disease mitochondrial hypothesis: where are we at? Neuroscientist. doi: 10.1177/1073858415574600
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–80
Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ (1983) A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A 80:4546–50
Smeyne RJ, Jackson-Lewis V (2005) The MPTP model of Parkinson’s disease. Brain Res Mol Brain Res 134:57–66. doi:10.1016/j.molbrainres.2004.09.017
Parker WD Jr, Boyson SJ, Parks JK (1989) Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol 26:719–23. doi:10.1002/ana.410260606
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1:1269
Ransom BR, Kunis DM, Irwin I, Langston JW (1987) Astrocytes convert the parkinsonism inducing neurotoxin, MPTP, to its active metabolite, MPP+. Neurosci Lett 75:323–8
Beal MF (2001) Experimental models of Parkinson’s disease. Nat Rev Neurosci 2:325–34. doi:10.1038/35072550
Vila M, Przedborski S (2003) Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci 4:365–75. doi:10.1038/nrn1100
Prediger RD, Batista LC, Medeiros R, Pandolfo P, Florio JC, Takahashi RN (2006) The risk is in the air: intranasal administration of MPTP to rats reproducing clinical features of Parkinson’s disease. Exp Neurol 202:391–403. doi:10.1016/j.expneurol.2006.07.001
Prediger RD, Aguiar AS Jr, Moreira EL, Matheus FC, Castro AA, Walz R, De Bem AF, Latini A, Tasca CI, Farina M, Raisman-Vozari R (2011) The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson’s disease. Curr Pharm Des 17:489–507
Ghosh A, Roy A, Matras J, Brahmachari S, Gendelman HE, Pahan K (2009) Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurosci 29:13543–56. doi:10.1523/JNEUROSCI.4144-09.2009
Roy A, Pahan K (2011) Prospects of statins in Parkinson disease. Neuroscientist 17:244–55. doi:10.1177/1073858410385006
Yan J, Xu Y, Zhu C, Zhang L, Wu A, Yang Y, **ong Z, Deng C, Huang XF, Yenari MA, Yang YG, Ying W, Wang Q (2011) Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: the association with anti-inflammatory responses. PLoS One 6:e20945. doi:10.1371/journal.pone.0020945
Bar-On P, Crews L, Koob AO, Mizuno H, Adame A, Spencer B, Masliah E (2008) Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson’s disease. J Neurochem 105:1656–67. doi:10.1111/j.1471-4159.2008.05254.x
Wang Q, Yan J, Chen X, Li J, Yang Y, Weng J, Deng C, Yenari MA (2011) Statins: multiple neuroprotective mechanisms in neurodegenerative diseases. Exp Neurol 230:27–34. doi:10.1016/j.expneurol.2010.04.006
Colle D, Santos DB, Moreira EL, Hartwig JM, dos Santos AA, Zimmermann LT, Hort MA, Farina M (2013) Probucol increases striatal glutathione peroxidase activity and protects against 3-nitropropionic acid-induced pro-oxidative damage in rats. PLoS One 8:e67658. doi:10.1371/journal.pone.0067658
Santos DB, Colle D, Moreira EL, Peres KC, Ribeiro RP, dos Santos AA, de Oliveira J, Hort MA, de Bem AF, Farina M (2015) Probucol mitigates streptozotocin-induced cognitive and biochemical changes in mice. Neuroscience 284:590–600. doi:10.1016/j.neuroscience.2014.10.019
Santos DB, Peres KC, Ribeiro RP, Colle D, dos Santos AA, Moreira EL, Souza DO, Figueiredo CP, Farina M (2012) Probucol, a lipid-lowering drug, prevents cognitive and hippocampal synaptic impairments induced by amyloid beta peptide in mice. Exp Neurol 233:767–75. doi:10.1016/j.expneurol.2011.11.036
Colle D, Santos DB, Hartwig JM, Godoi M, Braga AL, Farina M (2013) Succinobucol versus probucol: higher efficiency of succinobucol in mitigating 3-NP-induced brain mitochondrial dysfunction and oxidative stress in vitro. Mitochondrion 13:125–33. doi:10.1016/j.mito.2013.01.005
Poirier J, Miron J, Picard C, Gormley P, Theroux L, Breitner J, Dea D (2014) Apolipoprotein E and lipid homeostasis in the etiology and treatment of sporadic Alzheimer’s disease. Neurobiol Aging 35(Suppl 2):S3–10. doi:10.1016/j.neurobiolaging.2014.03.037
Endo K, Saiki A, Yamaguchi T, Sakuma K, Sasaki H, Ban N, Kawana H, Nagayama D, Nagumo A, Ohira M, Oyama T, Murano T, Miyashita Y, Yamamura S, Suzuki Y, Shirai K, Tatsuno I (2013) Probucol suppresses initiation of chronic hemodialysis therapy and renal dysfunction-related death in diabetic nephropathy patients: Sakura study. J Atheroscler Thromb 20:494–502
Kasai T, Miyauchi K, Kubota N, Kajimoto K, Amano A, Daida H (2012) Probucol therapy improves long-term (>10-year) survival after complete revascularization: a propensity analysis. Atherosclerosis 220:463–9. doi:10.1016/j.atherosclerosis.2011.09.051
Yamashita S, Hbujo H, Arai H, Harada-Shiba M, Matsui S, Fukushima M, Saito Y, Kita T, Matsuzawa Y (2008) Long-term probucol treatment prevents secondary cardiovascular events: a cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb 15:292–303
Kunsch C, Luchoomun J, Grey JY, Olliff LK, Saint LB, Arrendale RF, Wasserman MA, Saxena U, Medford RM (2004) Selective inhibition of endothelial and monocyte redox-sensitive genes by AGI-1067: a novel antioxidant and anti-inflammatory agent. J Pharmacol Exp Ther 308:820–9. doi:10.1124/jpet.103.059733
Muldrew KM, Franks AM (2009) Succinobucol: review of the metabolic, antiplatelet and cardiovascular effects. Expert Opin Investig Drugs 18:531–9. doi:10.1517/13543780902849244
Sundell CL, Somers PK, Meng CQ, Hoong LK, Suen KL, Hill RR, Landers LK, Chapman A, Butteiger D, Jones M, Edwards D, Daugherty A, Wasserman MA, Alexander RW, Medford RM, Saxena U (2003) AGI-1067: a multifunctional phenolic antioxidant, lipid modulator, anti-inflammatory and antiatherosclerotic agent. J Pharmacol Exp Ther 305:1116–23. doi:10.1124/jpet.102.048132
Colle D, Santos DB, Hartwig JM, Godoi M, Engel DF, de Bem AF, Braga AL and Farina M (2015) Succinobucol, a lipid-lowering drug, protects against 3-nitropropionic acid-induced mitochondrial dysfunction and oxidative stress in SH-SY5Y cells via upregulation of glutathione levels and glutamate cysteine ligase activity. Mol Neurobiol. doi: 10.1007/s12035-014-9086-x
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–97. doi:10.1016/S1474-4422(09)70062-6
Tsang AH, Chung KK (2009) Oxidative and nitrosative stress in Parkinson’s disease. Biochim Biophys Acta 1792:643–50. doi:10.1016/j.bbadis.2008.12.006
Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Anderson TJ, Reeves F, Title LM, Schampaert E, LeMay M, Lesperance J, Scott R, Guertin MC, Brennan ML, Hazen SL, Bertrand OF (2008) Effects of the antioxidant succinobucol (AGI-1067) on human atherosclerosis in a randomized clinical trial. Atherosclerosis 197:480–6. doi:10.1016/j.atherosclerosis.2006.11.039
Solari N, Bonito-Oliva A, Fisone G, Brambilla R (2013) Understanding cognitive deficits in Parkinson’s disease: lessons from preclinical animal models. Learn Mem 20:592–600. doi:10.1101/lm.032029.113
Weingarten D (2004) Process of preparing esters and ethers of probucol and derivatives thereof., Patent WO2004062622
Siveski-Iliskovic N, Hill M, Chow DA, Singal PK (1995) Probucol protects against adriamycin cardiomyopathy without interfering with its antitumor effect. Circulation 91:10–5
Castro AA, Wiemes BP, Matheus FC, Lapa FR, Viola GG, Santos AR, Tasca CI, Prediger RD (2013) Atorvastatin improves cognitive, emotional and motor impairments induced by intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in rats, an experimental model of Parkinson’s disease. Brain Res 1513:103–16. doi:10.1016/j.brainres.2013.03.029
Moreira EL, Rial D, Aguiar AS Jr, Figueiredo CP, Siqueira JM, DalBo S, Horst H, de Oliveira J, Mancini G, dos Santos TS, Villarinho JG, Pinheiro FV, Marino-Neto J, Ferreira J, De Bem AF, Latini A, Pizzolatti MG, Ribeiro-do-Valle RM, Prediger RD (2010) Proanthocyanidin-rich fraction from Croton celtidifolius Baill confers neuroprotection in the intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine rat model of Parkinson’s disease. J Neural Transm 117:1337–51. doi:10.1007/s00702-010-0464-x
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8:464–74. doi:10.1016/S1474-4422(09)70068-7
Liepelt-Scarfone I, Behnke S, Godau J, Schweitzer KJ, Wolf B, Gaenslen A, Berg D (2011) Relation of risk factors and putative premotor markers for Parkinson’s disease. J Neural Transm 118:579–85. doi:10.1007/s00702-010-0553-x
Morley JF, Pawlowski SM, Kesari A, Maina I, Pantelyat A, Duda JE (2014) Motor and non-motor features of Parkinson’s disease that predict persistent drug-induced Parkinsonism. Parkinsonism Relat Disord 20:738–42. doi:10.1016/j.parkreldis.2014.03.024
Dantzer R, Bluthe RM, Koob GF, Le Moal M (1987) Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 91:363–8
Moreira EL, de Oliveira J, Nunes JC, Santos DB, Nunes FC, Vieira DS, Ribeiro-do-Valle RM, Pamplona FA, de Bem AF, Farina M, Walz R, Prediger RD (2012) Age-related cognitive decline in hypercholesterolemic LDL receptor knockout mice (LDLr−/−): evidence of antioxidant imbalance and increased acetylcholinesterase activity in the prefrontal cortex. J Alzheimers Dis 32:495–511. doi:10.3233/JAD-2012-120541
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–16. doi:10.1006/abbi.1996.0178
Latini A, da Silva CG, Ferreira GC, Schuck PF, Scussiato K, Sarkis JJ, Dutra Filho CS, Wyse AT, Wannmacher CM, Wajner M (2005) Mitochondrial energy metabolism is markedly impaired by D-2-hydroxyglutaric acid in rat tissues. Mol Genet Metab 86:188–99. doi:10.1016/j.ymgme.2005.05.002
Matsumoto T, Suzuki O, Furuta T, Asai M, Kurokawa Y, Nimura Y, Katsumata Y, Takahashi I (1985) A sensitive fluorometric assay for serum monoamine oxidase with kynuramine as substrate. Clin Biochem 18:126–9
Naoi M, Nomura Y, Ishiki R, Suzuki H, Nagatsu T (1998) 4-(O-benzylphenoxy)-N-methylbutylamine (bifemelane) and other 4-(O-benzylphenoxy)-N-methylalkyl amines as new inhibitors of type B monoamine oxidase. J Neurochem 50:243–247
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–75
Douhou A, Troadec JD, Ruberg M, Raisman-Vozari R, Michel PP (2001) Survival promotion of mesencephalic dopaminergic neurons by depolarizing concentrations of K+ requires concurrent inactivation of NMDA or AMPA/kainate receptors. J Neurochem 78:163–74
Kawamoto JC, Barrett JN (1986) Cryopreservation of primary neurons for tissue culture. Brain Res 384:84–93
Guerreiro S, Toulorge D, Hirsch E, Marien M, Sokoloff P, Michel PP (2008) Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol Pharmacol 74:980–9. doi:10.1124/mol.108.048207
Traver S, Marien M, Martin E, Hirsch EC, Michel PP (2006) The phenotypic differentiation of locus ceruleus noradrenergic neurons mediated by brain-derived neurotrophic factor is enhanced by corticotropin releasing factor through the activation of a cAMP-dependent signaling pathway. Mol Pharmacol 70:30–40. doi:10.1124/mol.106.022715
Lotharius J, O’Malley KL (2000) The parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J Biol Chem 275:38581–8. doi:10.1074/jbc.M005385200
Richardson JR, Caudle WM, Guillot TS, Watson JL, Nakamaru-Ogiso E, Seo BB, Sherer TB, Greenamyre JT, Yagi T, Matsuno-Yagi A, Miller GW (2007) Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Toxicol Sci 95:196–204. doi:10.1093/toxsci/kfl133
Stojkovska I, Wagner BM and Morrison BE (2015) Parkinson’s disease and enhanced inflammatory response. Exp Biol Med (Maywood). doi: 10.1177/1535370215576313
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–34. doi:10.1007/s00441-004-0956-9
Tardif JC, Gregoire J, Schwartz L, Title L, Laramee L, Reeves F, Lesperance J, Bourassa MG, L’Allier PL, Glass M, Lambert J, Guertin MC (2003) Effects of AGI-1067 and probucol after percutaneous coronary interventions. Circulation 107:552–8
Ribeiro RP, Moreira EL, Santos DB, Colle D, Dos Santos AA, Peres KC, Figueiredo CP, Farina M (2013) Probucol affords neuroprotection in a 6-OHDA mouse model of Parkinson’s disease. Neurochem Res 38:660–8. doi:10.1007/s11064-012-0965-0
Rojo AI, Montero C, Salazar M, Close RM, Fernandez-Ruiz J, Sanchez-Gonzalez MA, de Sagarra MR, Jackson-Lewis V, Cavada C, Cuadrado A (2006) Persistent penetration of MPTP through the nasal route induces Parkinson’s disease in mice. Eur J Neurosci 24:1874–84. doi:10.1111/j.1460-9568.2006.05060.x
Doty RL (2012) Olfactory dysfunction in Parkinson disease. Nat Rev Neurol 8:329–39. doi:10.1038/nrneurol.2012.80
Schintu N, Frau L, Ibba M, Garau A, Carboni E, Carta AR (2009) Progressive dopaminergic degeneration in the chronic MPTPp mouse model of Parkinson’s disease. Neurotox Res 16:127–39. doi:10.1007/s12640-009-9061-x
Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM (2003) Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci 23:6351–6
Bove J, Perier C (2012) Neurotoxin-based models of Parkinson’s disease. Neuroscience 211:51–76. doi:10.1016/j.neuroscience.2011.10.057
Przedborski S, Vila M (2003) The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: a tool to explore the pathogenesis of Parkinson’s disease. Ann N Y Acad Sci 991:189–98
Jackson-Lewis V, Przedborski S (2007) Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc 2:141–51. doi:10.1038/nprot.2006.342
Kolacheva AA, Kozina EA, Volina EV, Ugryumov MV (2014) Time course of degeneration of dopaminergic neurons and respective compensatory processes in the nigrostriatal system in mice. Dokl Biol Sci 456:160–4. doi:10.1134/S0012496614030041
Wu X, Cai H, Ge R, Li L, Jia Z (2014) Recent progress of imaging agents for Parkinson’s disease. Curr Neuropharmacol 12:551–63. doi:10.2174/1570159X13666141204221238
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27
Rachakonda V, Pan TH, Le WD (2004) Biomarkers of neurodegenerative disorders: how good are they? Cell Res 14:347–58. doi:10.1038/sj.cr.7290235
Storch A, Ludolph AC, Schwarz J (2004) Dopamine transporter: involvement in selective dopaminergic neurotoxicity and degeneration. J Neural Transm (Vienna) 111:1267–86. doi:10.1007/s00702-004-0203-2
Heikkila RE, Manzino L, Cabbat FS, Duvoisin RC (1984) Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature 311:467–9
Muralikrishnan D, Samantaray S, Mohanakumar KP (2003) D-deprenyl protects nigrostriatal neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity. Synapse 50:7–13. doi:10.1002/syn.10239
Currais A (2015) Ageing and inflammation—a central role for mitochondria in brain health and disease. Ageing Res Rev 21:30–42. doi:10.1016/j.arr.2015.02.001
Kim HK, Chen W, Andreazza AC (2015) The potential role of the NLRP3 inflammasome as a link between mitochondrial complex I dysfunction and inflammation in bipolar disorder. Neural Plast 2015:408136. doi:10.1155/2015/408136
Yamashita S, Matsuzawa Y (2009) Where are we with probucol: a new life for an old drug? Atherosclerosis 207:16–23. doi:10.1016/j.atherosclerosis.2009.04.002
Khan MM, Kempuraj D, Thangavel R, Zaheer A (2013) Protection of MPTP-induced neuroinflammation and neurodegeneration by Pycnogenol. Neurochem Int 62:379–88. doi:10.1016/j.neuint.2013.01.029
Samantaray S, Knaryan VH, Shields DC, Cox AA, Haque A, Banik NL (2015) Inhibition of calpain activation protects MPTP-induced nigral and spinal cord neurodegeneration, reduces inflammation, and improves gait dynamics in mice. Mol Neurobiol 52:1054–66. doi:10.1007/s12035-015-9255-6
Muzerengi S, Contrafatto D, Chaudhuri KR (2007) Non-motor symptoms: identification and management. Parkinsonism Relat Disord 13(Suppl 3):S450–6. doi:10.1016/S1353-8020(08)70048-8
Calabresi P, Castrioto A, Di Filippo M, Picconi B (2013) New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson’s disease. Lancet Neurol 12:811–21. doi:10.1016/S1474-4422(13)70118-2
Moriguchi S, Yabuki Y, Fukunaga K (2012) Reduced calcium/calmodulin-dependent protein kinase II activity in the hippocampus is associated with impaired cognitive function in MPTP-treated mice. J Neurochem 120:541–51. doi:10.1111/j.1471-4159.2011.07608.x
Baddeley AD, Hitch GJ (1974) Working memory. In: Recent advances in learning and motivation 8., pp 47–90
Baddeley A (1986) Modularity, mass-action and memory. Q J Exp Psychol A 38:527–33
Acknowledgments
The authors gratefully thank the financial support and grants provided by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and CAPES-COFECUB (France/Brazil; 681/2010). RRV and PPM were supported by program Investissements d’avenir ANR-10-IAIHU-06.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The procedures used in the present study were approved by the Committee of Ethics for the Use of Animals—Universidade Federal de Santa Catarina (CEUA/UFSC; PP00546). Animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996), European Directive 86/609, and the National Institutes of Health (NIH) publication “Principles of Laboratory Animal Care.”
Conflict of Interest
The authors have no financial or personal conflict of interest related to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Scheme of in vivo experimental protocols. A) Experimental protocol 1; B) Experimental protocol 2 and C) Experimental protocol 3. (GIF 24 kb)
Supplemental Figure 2
Effects of succinobucol and/or MPTP treatment on tyrosine hydroxylase (TH) and dopamine transporter (DAT) levels in the striatum. (A) Representative images of TH immunostaining in the striatum (scale bar = 2 mm). (B) Relative quantification of the optical density TH in striatum of mice. (C) TH protein content measured by Western Blot analysis. (D) Representative images of DAT immunostaining in the striatum (scale bar = 500 μm). (E) Relative quantification of the optical density DAT neurons in striatum of mice. Values represent the mean ± SEM (n = 5 animals/group). *p < 0.05; ***p < 0.001 compared to the control group; #p < 0.05; ###p < 0.001 compared to the MPTP group (two-way ANOVA followed by Newman- Keuls test). (GIF 8 kb)
Supplemental Figure 3
Effects of succinobucol (10 mg/kg/day, i.g.; during 45 consecutive days) on glial fibrillary acidic protein (GFAP) in the striatum evaluated through immunohistochemistry 15 days after i.n. infusion of control (saline) or MPTP (1 mg/nostril). (A) Representative images of GFAP immunostaining in the striatum (scale bar = 2 mm). (B) Relatives quantifications of the GFAP optical densities in striatum. Values represent the mean ± SEM (n = 5 animals/group). ***p < 0.001 compared to the control group; ##p < 0.01, compared to the MPTP group (two-way ANOVA followed by Newman- Keuls test). (GIF 2 kb)
Supplementary table 1
Succinobucol (SUC) reduces plasma cholesterol levels in mice. Animals were treated with SUC and/or MPTP according to Supplemental Figure 1. Plasma cholesterol levels are expressed as mg/dL and presented as mean ± S.E.M. (n = 6-7 mice/group). **p < 0.01 compared to the control group; ##p < 0.01, compared to the MPTP group (two-way ANOVA followed by Newman- Keuls test). (DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Santos, D.B., Colle, D., Moreira, E.L.G. et al. Succinobucol, a Non-Statin Hypocholesterolemic Drug, Prevents Premotor Symptoms and Nigrostriatal Neurodegeneration in an Experimental Model of Parkinson’s Disease. Mol Neurobiol 54, 1513–1530 (2017). https://doi.org/10.1007/s12035-016-9747-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9747-z