Abstract
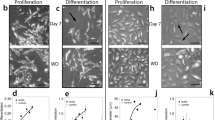
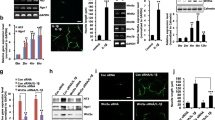
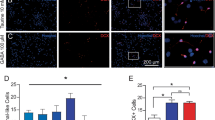
We investigated the role of neuronal nitric oxide synthase (nNOS) in the neuronal differentiation of neural progenitor cells (NPCs) from hippocampi of E16.5 rat embryos. The production of nitric oxide (NO) and nNOS expression increased markedly during neuronal differentiation as did the expression of neurotrophin-3 (NT3), neurotrophin-4/5 (NT 4/5), and synapsin I. nNOS siRNA or the nNOS inhibitor, 7-nitroindazole (7-NI), decreased expression of the neurotrophins and synapsin I, and suppressed neurite outgrowth. These results suggest that nNOS plays a critical role in neuronal differentiation of hippocampal NPCs. nNOS-mediated neuronal differentiation is controlled by calcineurin since cyclosporin A (CsA), a calcineurin inhibitor, decreased nNOS activation and NO production, and inhibited neurite outgrowth. We found that inactivation of glycogen synthase kinase-3 beta (GSK3β) resulting from activation of protein kinase C alpha (PKCα) is involved in the nNOS-mediated neuronal differentiation. Moreover, lithium chloride (LiCl), a GSK3β inhibitor, increased neuronal differentiation by inhibiting the proliferation of NPCs. Taken together, these results suggest that neuronal differentiation is dependent on calcineurin-mediated activation of nNOS; this induces PKCα-dependent inactivation of GSK3β, which leads to inhibition of the proliferation of hippocampal NPCs.





Similar content being viewed by others
References
Masliukov PM, Emanuilov AI, Madalieva LV, Moiseev KY, Bulibin AV, Korzina MB, Porseva VV, Korobkin AA, et al. (2014) Development of nNOS-positive neurons in the rat sensory and sympathetic ganglia. Neuroscience 256:271–281. doi:10.1016/j.neuroscience.2013.10.013
Tricoire L, Vitalis T (2012) Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits. Frontiers in neural circuits 6:82. doi:10.3389/fncir.2012.00082
Park SY, Koh YJ, Cho JH, Oh DY, Shin SA, Lee KS, Lee HB, Han JS (2007) Nicotine inhibits bFGF-induced neurite outgrowth through suppression of NO synthesis in H19-7 cells. Neurochem Res 32(3):481–488. doi:10.1007/s11064-006-9256-y
Niu L, Chen T, Wang YY, Li YQ (2009) Neurochemical phenotypes of endomorphin-2-containing neurons in vagal nodose neurons of the adult rat. Neurochem Int 55(7):542–551. doi:10.1016/j.neuint.2009.05.010
Sanchez-Islas E, Leon-Olea M (2004) Nitric oxide synthase inhibition during synaptic maturation decreases synapsin I immunoreactivity in rat brain. Nitric Oxide 10(3):141–149. doi:10.1016/j.niox.2004.04.001
Virgili M, Monti B, LoRusso A, Bentivogli M, Contestabile A (1999) Developmental effects of in vivo and in vitro inhibition of nitric oxide synthase in neurons. Brain Res 839(1):164–172
Ma SX, Peterson RG, Magee EM, Lee P, Lee WP, Li XY (2015) Impaired expression of neuronal nitric oxide synthase in the gracile nucleus is involved in neuropathic changes in Zucker diabetic fatty rats with and without 2,5-hexanedione intoxication. Neurosci Res. doi:10.1016/j.neures.2015.10.007
Fritzen S, Schmitt A, Koth K, Sommer C, Lesch KP, Reif A (2007) Neuronal nitric oxide synthase (NOS-I) knockout increases the survival rate of neural cells in the hippocampus independently of BDNF. Mol Cell Neurosci 35(2):261–271. doi:10.1016/j.mcn.2007.02.021
Adachi M, Abe M, Sasaki T, Kato H, Kasahara J, Araki T (2010) Role of inducible or neuronal nitric oxide synthase in neurogenesis of the dentate gyrus in aged mice. Metab Brain Dis 25(4):419–424. doi:10.1007/s11011-010-9224-8
Sun Y, ** K, Childs JT, **e L, Mao XO, Greenberg DA (2005) Neuronal nitric oxide synthase and ischemia-induced neurogenesis. J Cereb Blood Flow Metab 25(4):485–492. doi:10.1038/sj.jcbfm.9600049
Myslivecek J, Hassmannova J, Barcal J, Safanda J, Zalud V (1996) Inhibitory learning and memory in newborn rats influenced by nitric oxide. Neuroscience 71(2):299–312
Vercelli A, Garbossa D, Repici M, Biasiol S, Jhaveri S (2001) Role of nitric oxide in the development of retinal projections. Ital J Anat Embryol 106(2 Suppl 1):489–498
Vercelli A, Garbossa D, Biasiol S, Repici M, Jhaveri S (2000) NOS inhibition during postnatal development leads to increased ipsilateral retinocollicular and retinogeniculate projections in rats. Eur J Neurosci 12(2):473–490
Yoshimura T, Arimura N, Kaibuchi K (2006) Molecular mechanisms of axon specification and neuronal disorders. Ann N Y Acad Sci 1086:116–125. doi:10.1196/annals.1377.013
Garrido JJ, Simon D, Varea O, Wandosell F (2007) GSK3 alpha and GSK3 beta are necessary for axon formation. FEBS Lett 581(8):1579–1586. doi:10.1016/j.febslet.2007.03.018
Schlessinger K, McManus EJ, Hall A (2007) Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol 178(3):355–361. doi:10.1083/jcb.200701083
Etienne-Manneville S, Hall A (2003) Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 421(6924):753–756. doi:10.1038/nature01423
Plattner F, Angelo M, Giese KP (2006) The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem 281(35):25457–25465. doi:10.1074/jbc.M603469200
Lee SJ, Chung YH, Joo KM, Lim HC, Jeon GS, Kim D, Lee WB, Kim YS, et al. (2006) Age-related changes in glycogen synthase kinase 3beta (GSK3beta) immunoreactivity in the central nervous system of rats. Neurosci Lett 409(2):134–139. doi:10.1016/j.neulet.2006.09.026
Cuesto G, Jordan-Alvarez S, Enriquez-Barreto L, Ferrus A, Morales M, Acebes A (2015) GSK3beta inhibition promotes synaptogenesis in Drosophila and mammalian neurons. PLoS One 10(3):e0118475. doi:10.1371/journal.pone.0118475
Acebes A, Martin-Pena A, Chevalier V, Ferrus A (2011) Synapse loss in olfactory local interneurons modifies perception. J Neurosci 31(8):2734–2745. doi:10.1523/JNEUROSCI.5046-10.2011
Martin-Pena A, Acebes A, Rodriguez JR, Sorribes A, de Polavieja GG, Fernandez-Funez P, Ferrus A (2006) Age-independent synaptogenesis by phosphoinositide 3 kinase. J Neurosci 26(40):10199–10208. doi:10.1523/JNEUROSCI.1223-06.2006
Hur EM, Zhou FQ (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11(8):539–551. doi:10.1038/nrn2870
Moore SF, van den Bosch MT, Hunter RW, Sakamoto K, Poole AW, Hers I (2013) Dual regulation of glycogen synthase kinase 3 (GSK3)alpha/beta by protein kinase C (PKC)alpha and Akt promotes thrombin-mediated integrin alphaIIbbeta3 activation and granule secretion in platelets. J Biol Chem 288(6):3918–3928. doi:10.1074/jbc.M112.429936
De Montigny A, Elhiri I, Allyson J, Cyr M, Massicotte G (2013) NMDA reduces Tau phosphorylation in rat hippocampal slices by targeting NR2A receptors, GSK3beta, and PKC activities. Neural Plast 2013:261593. doi:10.1155/2013/261593
Park SY, Ma W, Yoon SN, Kang MJ, Han JS (2015) Phospholipase D1 increases Bcl-2 expression during neuronal differentiation of rat neural stem cells. Mol Neurobiol 51(3):1089–1102. doi:10.1007/s12035-014-8773-y
Talman V, Amadio M, Osera C, Sorvari S, Boije Af Gennas G, Yli-Kauhaluoma J, Rossi D, Govoni S, et al. (2013) The C1 domain-targeted isophthalate derivative HMI-1b11 promotes neurite outgrowth and GAP-43 expression through PKCalpha activation in SH-SY5Y cells. Pharmacol Res 73:44–54. doi:10.1016/j.phrs.2013.04.008
Han NL, Wen J, Lin Q, Tan PL, Liou YC, Sheu FS (2007) Proteomics analysis of the expression of neurogranin in murine neuroblastoma (Neuro-2a) cells reveals its involvement for cell differentiation. Int J Biol Sci 3(5):263–273
Kim JS, Chang MY, IT Y, Kim JH, Lee SH, Lee YS, Son H (2004) Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem 89(2):324–336. doi:10.1046/j.1471-4159.2004.02329.x
Markus A, Patel TD, Snider WD (2002) Neurotrophic factors and axonal growth. Curr Opin Neurobiol 12(5):523–531
Cockrell A, Laroux FS, Jourd'heuil D, Kawachi S, Gray L, Van der Heyde H, Grisham MB (1999) Role of inducible nitric oxide synthase in leukocyte extravasation in vivo. Biochem Biophys Res Commun 257(3):684–686. doi:10.1006/bbrc.1999.0484
Sachewsky N, Hunt J, Cooke MJ, Azimi A, Zarin T, Miu C, Shoichet MS, Morshead CM (2014) Cyclosporin A enhances neural precursor cell survival in mice through a calcineurin-independent pathway. Dis Model Mech 7(8):953–961. doi:10.1242/dmm.014480
Diaz-Ruiz A, Vergara P, Perez-Severiano F, Segovia J, Guizar-Sahagun G, Ibarra A, Rios C (2005) Cyclosporin-a inhibits constitutive nitric oxide synthase activity and neuronal and endothelial nitric oxide synthase expressions after spinal cord injury in rats. Neurochem Res 30(2):245–251
Zaragosi LE, Wdziekonski B, Fontaine C, Villageois P, Peraldi P, Dani C (2008) Effects of GSK3 inhibitors on in vitro expansion and differentiation of human adipose-derived stem cells into adipocytes. BMC Cell Biol 9:11. doi:10.1186/1471-2121-9-11
Jope RS, Johnson GV (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29(2):95–102. doi:10.1016/j.tibs.2003.12.004
Bijur GN, Jope RS (2003) Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport 14(18):2415–2419. doi:10.1097/01.wnr.0000099609.19426.70
Lenox RH, Wang L (2003) Molecular basis of lithium action: integration of lithium-responsive signaling and gene expression networks. Mol Psychiatry 8(2):135–144. doi:10.1038/sj.mp.4001306
Williams RS, Cheng L, Mudge AW, Harwood AJ (2002) A common mechanism of action for three mood-stabilizing drugs. Nature 417(6886):292–295. doi:10.1038/417292a
Chen RH, Ding WV, McCormick F (2000) Wnt signaling to beta-catenin involves two interactive components. Glycogen synthase kinase-3beta inhibition and activation of protein kinase C. J Biol Chem 275(23):17894–17899. doi:10.1074/jbc.M905336199
Contestabile A, Monti B, Contestabile A, Ciani E (2003) Brain nitric oxide and its dual role in neurodegeneration/neuroprotection: understanding molecular mechanisms to devise drug approaches. Curr Med Chem 10(20):2147–2174
Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, et al. (1996) Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84(5):757–767
Cheng A, Wang S, Cai J, Rao MS, Mattson MP (2003) Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol 258(2):319–333
Etherington SJ, Everett AW (2004) Postsynaptic production of nitric oxide implicated in long-term depression at the mature amphibian (Bufo marinus) neuromuscular junction. J Physiol 559(Pt 2):507–517. doi:10.1113/jphysiol.2004.066498
Kim WY, Snider WD (2011) Functions of GSK-3 signaling in development of the nervous system. Front Mol Neurosci 4:44. doi:10.3389/fnmol.2011.00044
Hall AC, Brennan A, Goold RG, Cleverley K, Lucas FR, Gordon-Weeks PR, Salinas PC (2002) Valproate regulates GSK-3-mediated axonal remodeling and synapsin I clustering in develo** neurons. Mol Cell Neurosci 20(2):257–270
Wang L, Zhu ZA (2014) Nitric oxide show its survival role by NO-PKC pathway through cGMP-dependent or independent on the culture of cerebella granular neurons. Neurosci Lett 583:165–169. doi:10.1016/j.neulet.2014.06.062
Perez-Rodriguez R, Olivan AM, Roncero C, Moron-Oset J, Gonzalez MP, Oset-Gasque MJ (2014) Glutamate triggers neurosecretion and apoptosis in bovine chromaffin cells through a mechanism involving NO production by neuronal NO synthase activation. Free Radic Biol Med 69:390–402. doi:10.1016/j.freeradbiomed.2014.01.029
Acknowledgments
We are grateful to Dr. Young-Myeong Kim (Department of Molecular and Cellular Biochemistry, College of Medicine, Kangwon National University, Chunchon, Kangwon-do, Republic of Korea) for the nNOS plasmid. This work was supported by a grant from the Medical Research Center program (NRF-2012R1A5A2A34671243) of the Ministry of Science and Technology, Republic of Korea, and partly supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (2015R1C1A1A02037376).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Figure 1
(PPTX 819 kb)
Rights and permissions
About this article
Cite this article
Park, SY., Kang, MJ. & Han, JS. Neuronal NOS Induces Neuronal Differentiation Through a PKCα-Dependent GSK3β Inactivation Pathway in Hippocampal Neural Progenitor Cells. Mol Neurobiol 54, 5646–5656 (2017). https://doi.org/10.1007/s12035-016-0110-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0110-1