Abstract
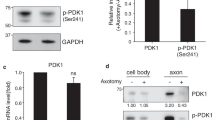
Following injury to peripheral axons, besides increased cyclic adenosine monophosphate (cAMP), the positive injury signals extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and signal transducer and activator of transcription 3 (STAT-3) are locally activated and retrogradely transported to the cell body, where they induce a pro-regenerative program. Here, to further understand the importance of injury signaling for successful axon regeneration, we used dorsal root ganglia (DRG) neurons that have a central branch without regenerative capacity and a peripheral branch that regrows after lesion. Although injury to the DRG central branch (dorsal root injury (DRI)) activated ERK, JNK, and STAT-3 and increased cAMP levels, it did not elicit gain of intrinsic growth capacity nor the ability to overcome myelin inhibition, as occurred after peripheral branch injury (sciatic nerve injury (SNI)). Besides, gain of growth capacity after SNI was independent of ERK and cAMP. Antibody microarrays of dynein-immunoprecipitated axoplasm from rats with either DRI or SNI revealed a broad differential activation and transport of signals after each injury type and further supported that ERK, JNK, STAT-3, and cAMP signaling pathways are minor contributors to the differential intrinsic axon growth capacity of both injury models. Increased levels of inhibitory injury signals including GSK3β and ROCKII were identified after DRI, not only in axons but also in DRG cell bodies. In summary, our work shows that activation and transport of positive injury signals are not sufficient to promote increased axon growth capacity and that differential modulation of inhibitory molecules may contribute to limited regenerative response.




Similar content being viewed by others
References
Devor M (1999) Unexplained peculiarities of the dorsal root ganglion. Pain Suppl 6:S27–S35. doi:10.1016/S0304-3959(99)00135-9
Smith DS, Skene JH (1997) A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci 17:646–658
Neumann S, Woolf CJ (1999) Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23:83–91
Beggah AT, Dours-Zimmermann MT, Barras FM, Brosius A, Zimmermann DR, Zurn AD (2005) Lesion-induced differential expression and cell association of Neurocan, Brevican, Versican V1 and V2 in the mouse dorsal root entry zone. Neuroscience 133:749–762. doi:10.1016/j.neuroscience.2005.03.005
Zhang Y, Tohyama K, Winterbottom JK, Haque NS, Schachner M, Lieberman AR, Anderson PN (2001) Correlation between putative inhibitory molecules at the dorsal root entry zone and failure of dorsal root axonal regeneration. Mol Cell Neurosci 17:444–459. doi:10.1006/mcne.2000.0952
Di Maio A, Skuba A, Himes BT, Bhagat SL, Hyun JK, Tessler A, Bishop D, Son YJ (2011) In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J Neurosci 31:4569–4582. doi:10.1523/JNEUROSCI.4638-10.2011
Seijffers R, Allchorne AJ, Woolf CJ (2006) The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci 32:143–154. doi:10.1016/j.mcn.2006.03.005
Schreyer DJ, Skene JH (1993) Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. J Neurobiol 24:959–970. doi:10.1002/neu.480240709
Mason MR, Lieberman AR, Grenningloh G, Anderson PN (2002) Transcriptional upregulation of SCG10 and CAP-23 is correlated with regeneration of the axons of peripheral and central neurons in vivo. Mol Cell Neurosci 20:595–615. doi:10.1006/mcne.2002.1140
Mar FM, Bonni A, Sousa MM (2014) Cell intrinsic control of axon regeneration. EMBO Rep 15:254–263. doi:10.1002/embr.201337723
Ambron RT, Dulin MF, Zhang XP, Schmied R, Walters ET (1995) Axoplasm enriched in a protein mobilized by nerve injury induces memory-like alterations in Aplysia neurons. J Neurosci 15:3440–3446
Ambron RT, Schmied R, Huang CC, Smedman M (1992) A signal sequence mediates the retrograde transport of proteins from the axon periphery to the cell body and then into the nucleus. J Neurosci 12:2813–2818
Cavalli V, Kujala P, Klumperman J, Goldstein LS (2005) Sunday Driver links axonal transport to damage signaling. J Cell Biol 168:775–787. doi:10.1083/jcb.200410136
Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M (2005) Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 45:715–726. doi:10.1016/j.neuron.2005.01.023
Barnat M, Enslen H, Propst F, Davis RJ, Soares S, Nothias F (2010) Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J Neurosci Off J Soc Neurosci 30:7804–7816. doi:10.1523/JNEUROSCI.0372-10.2010
Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F et al (2012) Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J 31:1350–1363. doi:10.1038/emboj.2011.494
Ambron RT, Zhang XP, Gunstream JD, Povelones M, Walters ET (1996) Intrinsic injury signals enhance growth, survival, and excitability of Aplysia neurons. J Neurosci 16:7469–7477
Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT (2001) Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci 21:4731–4739
Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT (2002) Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34:895–903. doi:10.1016/S0896-6273(02)00730-4
Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI (2002) Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 34:885–893. doi:10.1016/S0896-6273(02)00702-X
Blesch A, Lu P, Tsukada S, Alto LT, Roet K, Coppola G, Geschwind D, Tuszynski MH (2012) Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to cAMP-mediated effects. Exp Neurol 235:162–173. doi:10.1016/j.expneurol.2011.12.037
Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE et al (2002) Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci 3:16. doi:10.1186/1471-2202-3-16
Lindwall C, Kanje M (2005) Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol Cell Neurosci 29:269–282. doi:10.1016/j.mcn.2005.03.002
Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER et al (2004) Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron 44:609–621. doi:10.1016/j.neuron.2004.10.030
Ylera B, Erturk A, Hellal F, Nadrigny F, Hurtado A, Tahirovic S, Oudega M, Kirchhoff F, Bradke F (2009) Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr Biol 19:930–936. doi:10.1016/j.cub.2009.04.017
Miranda CO, Teixeira CA, Liz MA, Sousa VF, Franquinho F, Forte G, Di Nardo P, Pinto-Do OP, Sousa MM (2011) Systemic delivery of bone marrow-derived mesenchymal stromal cells diminishes neuropathology in a mouse model of Krabbe's disease. Stem Cells 29:1738–1751. doi:10.1002/stem.724
Mar FM, da Silva TF, Morgado MM, Rodrigues LG, Rodrigues D, Pereira MI, Marques A, Sousa VF, Coentro J et al (2015) Myelin lipids inhibit axon regeneration following spinal cord injury: a novel perspective for therapy. Mol Neurobiol. doi:10.1007/s12035-014-9072-3
Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J et al (2003) Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40:1095–1104. doi:10.1016/S0896-6273(03)00770-0
Cheadle C, Vawter MP, Freed WJ, Becker KG (2003) Analysis of microarray data using Z score transformation. J Mol Diagn 5:73–81. doi:10.1016/S1525-1578(10)60455-2
Saijilafu, Hur EM, Liu CM, Jiao Z, Xu WL, Zhou FQ (2013) PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat Commun 4:2690. doi:10.1038/ncomms3690
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I et al (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322:963–966. doi:10.1126/science.1161566
Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, ** D et al (2010) PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci 13:1075–1081. doi:10.1038/nn.2603
Christie KJ, Webber CA, Martinez JA, Singh B, Zochodne DW (2010) PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J Neurosci 30:9306–9315. doi:10.1523/JNEUROSCI.6271-09.2010
Dill J, Wang H, Zhou F, Li S (2008) Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci 28:8914–8928. doi:10.1523/JNEUROSCI.1178-08.2008
Liz MA, Mar FM, Santos TE, Pimentel HI, Marques AM, Morgado MM, Vieira S, Sousa VF, Pemble H et al (2014) Neuronal deletion of GSK3beta increases microtubule speed in the growth cone and enhances axon regeneration via CRMP-2 and independently of MAP1B and CLASP2. BMC Biol 12:47. doi:10.1186/1741-7007-12-47
Alabed YZ, Grados-Munro E, Ferraro GB, Hsieh SH, Fournier AE (2006) Neuronal responses to myelin are mediated by rho kinase. J Neurochem 96:1616–1625. doi:10.1111/j.1471-4159.2006.03670.x
Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K (2005) GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120:137–149. doi:10.1016/j.cell.2004.11.012
Somlyo AP, Somlyo AV (2000) Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522(Pt 2):177–185
Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G et al (2011) Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480:372–375. doi:10.1038/nature10594
Lang C, Bradley PM, Jacobi A, Kerschensteiner M, Bareyre FM (2013) STAT3 promotes corticospinal remodelling and functional recovery after spinal cord injury. EMBO Rep 14:931–937. doi:10.1038/embor.2013.117
Michaelevski I, Segal-Ruder Y, Rozenbaum M, Medzihradszky KF, Shalem O, Coppola G, Horn-Saban S, Ben-Yaakov K, Dagan SY et al (2010) Signaling to transcription networks in the neuronal retrograde injury response. Sci Signal 3:ra53. doi:10.1126/scisignal.2000952
Amano M, Nakayama M, Kaibuchi K (2010) Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 67:545–554. doi:10.1002/cm.20472
Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L (2002) Rho signaling pathway targeted to promote spinal cord repair. J Neurosci 22:6570–6577
Duffy P, Schmandke A, Schmandke A, Sigworth J, Narumiya S, Cafferty WB, Strittmatter SM (2009) Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J Neurosci 29:15266–15276. doi:10.1523/JNEUROSCI.4650-09.2009
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617–627. doi:10.1038/nrn1956
Bijur GN, Jope RS (2003) Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport 14:2415–2419. doi:10.1097/01.wnr.0000099609.19426.70
Park KK, Liu K, Hu Y, Kanter JL, He Z (2010) PTEN/mTOR and axon regeneration. Exp Neurol 223:45–50. doi:10.1016/j.expneurol.2009.12.032
Acknowledgments
This work was funded by FEDER through COMPETE and by National funds through FCT—Fundação para a Ciência e a Tecnologia—under the Project FCOMP-01-0124-FEDER-017455 (HMSP ICT/0020/2010). F.M.M. is supported by FCT (SFRH/BPD/104503/2014). We thank Dr. Paula Sampaio (IBMC) for help with microscopy, Dr. Pedro Brites (IBMC) for providing myelin and for help in the analysis of the arrays, and Vera Sousa and Francisco Figueiredo for tissue processing.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Vip is increased following SNI and DRI. Representative images of immunohistochemistry against Vip in L4,5 DRG from naïve animals or animals with either SNI or DRI; scale bar 20 μm. (JPEG 18 kb)
Supplementary Fig. 2
PTEN and PKCα are not altered upon SNI or DRI. Representative images of immunohistochemistry against PTEN in L4,5 DRG from naïve animals or animals with either SNI or DRI; scale bar 20 μm (a). Quantification of a (b). Representative images of immunohistochemistry against PKCα in L4,5 DRG from naïve animals or animals with either SNI or DRI; scale bar 20 μm (c). Quantification of c (d). Results represent the mean +/− SEM. (JPEG 35 kb)
Supplementary Table 1
Summary of the analysis of the Kinex™ Antibody Microarray. All the protein targets analyzed are listed (some targets were covered by multiple antibodies present in the microarray—not shown). Targets with a Z ratio > +/−1.0 (i.e., that met selection criteria), the Z ratio value is shown. For targets that did not meet the selection criteria, the Z ratio is indicated as ns (non-significant). (XLSX 35 kb)
Rights and permissions
About this article
Cite this article
Mar, F.M., Simões, A.R., Rodrigo, I.S. et al. Inhibitory Injury Signaling Represses Axon Regeneration After Dorsal Root Injury. Mol Neurobiol 53, 4596–4605 (2016). https://doi.org/10.1007/s12035-015-9397-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9397-6