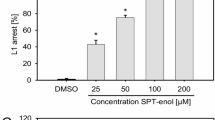
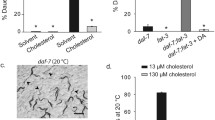
Abstract
Plant-parasitic nematodes ingest and convert host phytosterols via dealkylation to cholesterol for both structural and hormonal requirements. The insect 24-dehydrocholesterol reductase (DHCR24) was shown in vitro as a committed enzyme in the dealkylation via chemical blocking. However, an increased brood size and ovulation rate, instead compromised development, were observed in the engineered nematode Caenorhabditis elegans where the DHCR24 gene was knocked down, indicating the relationship between DHCR24 and dealkylation and their function in nematodes remains illusive. In this study, a defect in C. elegans DHCR24 causes impaired growth of the nematode with sitosterol (a major component of phytosterols) as a sole sterol source. Plant sterols with rationally designed structure (null substrates for dealkylation) can’t be converted to cholesterol in wild-type worms, and their development was completely halted. This study underpins the essential function of DHCR24 in nematodes and would be beneficial for the development of novel nematocidal strategies.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Gan, Q., Wang, X., Wang, Y., **e, Z., Tian, Y., & Lu, Y. (2017). Culture-free detection of crop pathogens at the single-cell level by micro-Raman spectroscopy. Advanced Science, 7, 1700127.
Nicol, J. M., Turner, S. J., Coyne, D. L., Nijs, Ld., Hockland, S., & Maafi, Z. T. (2011). Current nematode threats to world agriculture. In J. Jones, G. Gheysen, & C. Fenoll (Eds.), Genomics and molecular genetics of plant-nematode interactions (pp. 21–43). Dordrecht: Springer, Netherlands.
Abad, P., Gouzy, J., Aury, J.-M., Castagnone-Sereno, P., Danchin, E. G. J., Deleury, E., Perfus-Barbeoch, L., Anthouard, V., Artiguenave, F., Blok, V. C., et al. (2008). Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nature Biotechnology, 26, 909–915.
Nes, D. (2011). W: Biosynthesis of cholesterol and other sterols. Chemical Reviews, 111, 6423–6451.
Kurzchalia, T. V., & Ward, S. (2003). Why do worms need cholesterol? Nature Cell Biology, 5, 684–688.
Zhou, W., Fisher, P. M., Vanderloop, B. H., Shen, Y., Shi, H., Maldonado, A. J., Leaver, D. J., & Nes, W. D. (2020). A nematode sterol C4α-methyltransferase catalyzes a new methylation reaction responsible for sterol diversity. Journal of Lipid Research, 61, 192–204.
Fisher, P. M., Zhou, W., Miller, M. B., Shen, Y., Shi, H., & Nes, W. D. (2017). Substrate Specificity and Unusual Reaction Mechanism of the Sterol 4-Methyltransferase in Caenorhabdtis elegans. The FASEB Journal, 31, 629.622-629.622.
Zhou, W., Nguyen, H. T., & Nes, W. D. (2008). Plant sterol methyltransferases: Phytosterolomic analysis, enzymology, and bioengineering strategies. In advances in plant biochemistry and Molecular Biology. Pergamon, 1, 241–281.
Nes, W. D., Sinha, A., Jayasimha, P., Zhou, W., Song, Z., & Dennis, A. L. (2006). Probing the sterol binding site of soybean sterol methyltransferase by site-directed mutagenesis: Functional analysis of conserved aromatic amino acids in Region 1. Archives of Biochemistry and Biophysics, 448, 23–30.
Song, Z., Zhou, W., Liu, J., & Nes, W. D. (2004). Mechanism-based active site modification of the soybean sterol methyltransferase by 26,27-dehydrocycloartenol. Bioorganic and Medicinal Chemistry Letters, 14, 33–36.
Zhou, W., & David Nes, W. (2003). Sterol methyltransferase2: Purification, properties, and inhibition. Archives of Biochemistry and Biophysics, 420, 18–34.
Nes, W. D., Song, Z., Dennis, A. L., Zhou, W., Nam, J., & Miller, M. B. (2003). Biosynthesis of phytosterols: Kinetic mechanism for the enzymatic C-methylation of sterols. Journal of Biological Chemistry, 278, 34505–34516.
Diener, A. C., Li, H., Zhou, W., Whoriskey, W. J., Nes, W. D., & Fink, G. R. (2000). Sterol methyltransferase 1 controls the level of cholesterol in plants. The Plant Cell, 12, 853–870.
Lu, Y., Zhou, W., Wei, L., Li, J., Jia, J., Li, F., Smith, S. M., & Xu, J. (2014). Regulation of the cholesterol biosynthetic pathway and its integration with fatty acid biosynthesis in the oleaginous microalga Nannochloropsis oceanica. Biotechnology for Biofuels, 7, 81.
Lu, Y., Jiang, J., Zhao, H., Han, X., **ang, Y., & Zhou, W. (2020). Clade-specific sterol metabolites in dinoflagellate endosymbionts are associated with coral bleaching in response to environmental cues. mSystems. https://doi.org/10.1128/mSystems.00765-00720
Jiang, J., Wang, A., Deng, X., Zhou, W., Gan, Q., & Lu, Y. (2021). How Symbiodiniaceae meets the challenges of life during coral bleaching. Coral Reefs, 40, 1339–1353.
Zhou, W., Zhang, X., Wang, A., Yang, L., Gan, Q., Yi, L., Summons, R. E., Volkman, J. K., & Lu, Y. (2022). Widespread sterol methyltransferase participates in the biosynthesis of both C4α- and C4β-methyl sterols. Journal of the American Chemical Society. https://doi.org/10.1021/jacs.2c01401
Zhou, W., Warrilow, A. G. S., Thomas, C. D., Ramos, E., Parker, J. E., Price, C. L., Vanderloop, B. H., Fisher, P. M., Loftis, M. D., Kelly, D. E., et al. (2018). Functional importance for developmental regulation of sterol biosynthesis in Acanthamoeba castellanii. Biochimica et Biophysica Acta (BBA)-Moslecular and Cell Biology of Lipids, 1863, 1164–1178.
Lepesheva, G. I., Zaitseva, N. G., Nes, W. D., Zhou, W., Arase, M., Liu, J., Hill, G. C., & Waterman, M. R. (2006). CYP51 from Trypanosoma cruzi: A phyla-specific residue in the b’ helix defines substrate preferences of sterol 14α-demethylase. Journal of Biological Chemistry, 281, 3577–3585.
Nes, W. D., Jayasimha, P., Zhou, W., Kanagasabai, R., **, C., Jaradat, T. T., Shaw, R. W., & Bujnicki, J. M. (2004). Sterol methyltransferase: Functional analysis of highly conserved residues by site-directed mutagenesis. Biochemistry, 43, 569–576.
Lepesheva, G. I., Nes, W. D., Zhou, W., Hill, G. C., & Waterman, M. R. (2004). CYP51 from Trypanosoma brucei is obtusifoliol-specific. Biochemistry, 43, 10789–10799.
Hargrove, T. Y., Wawrzak, Z., Liu, J., Nes, W. D., Waterman, M. R., & Lepesheva, G. I. (2011). Substrate preferences and catalytic parameters determined by structural characteristics of sterol 14alpha-demethylase (CYP51) from Leishmania infantum. Journal of Biological Chemistry, 286, 26838–26848.
Zhou, W., Nguyen, T. T., Collins, M. S., Cushion, M. T., & Nes, W. D. (2002). Evidence for multiple sterol methyl transferase pathways in Pneumocystis carinii. Lipids, 37, 1177–1186.
Najle, S. R., Molina, M. C., Ruiz-Trillo, I., & Uttaro, A. D. (2016). Sterol metabolism in the filasterean Capsaspora owczarzaki has features that resemble both fungi and animals. Open Biologys, 6. https://doi.org/10.1098/rsob.160029
Gan, Q., Zhou, W., Wang, S., Li, X., **e, Z., Wang, J., Jiang, J., & Lu, Y. (2017). A customized contamination controlling approach for culturing oleaginous Nannochloropsis oceanica. Algal Research, 27, 376–382.
Nes, W. D., Zhou, W., Ganapathy, K., Liu, J., Vatsyayan, R., Chamala, S., Hernandez, K., & Miranda, M. (2009). Sterol 24-C-methyltransferase: An enzymatic target for the disruption of ergosterol biosynthesis and homeostasis in Cryptococcus neoformans. Archives of Biochemistry and Biophysics, 481, 210–218.
Zhou, W., Song, Z., Kanagasabai, R., Liu, J., Jayasimha, P., Sinha, A., Veeramachanemi, P., Miller, M. B., & Nes, W. D. (2004). Mechanism-based enzyme inactivators of phytosterol biosynthesis. Molecules, 9, 185–203.
Kanagasabai, R., Zhou, W., Liu, J., Nguyen, T. T., Veeramachaneni, P., & Nes, W. D. (2004). Disruption of ergosterol biosynthesis, growth, and the morphological transition in Candida albicans by sterol methyltransferase inhibitors containing sulfur at C-25 in the sterol side chain. Lipids, 39, 737–746.
Nes, W. D. (2000). Sterol methyl transferase: enzymology and inhibition. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1529, 63–88.
Zhou, W., Ramos, E., Zhu, X., Fisher, P. M., Kidane, M. E., Vanderloop, B. H., Thomas, C. D., Yan, J., Singha, U., Chaudhuri, M., et al. (2019). Steroidal antibiotics are antimetabolites of Acanthamoeba steroidogenesis with phylogenetic implications. Journal of Lipid Research, 60, 981–994.
Vanderloop, B. H., Thomas, C. D., Zhou, W., Gillespie, A. A., Singha, U., Chaudhuri, M., Neelam, S., Niederkorn, J., & Nes, W. D. (2019). Deregulation of Acanthamoeba castellanii steroidogenesis is amoebicidal and protects cultured corneal cells from Ac attack. The FASEB Journal, 33, 634.615-634.615.
Zhou, W., Debnath, A., Jennings, G., Hahn, H. J., Vanderloop, B. H., Chaudhuri, M., Nes, W. D., & Podust, L. M. (2018). Enzymatic chokepoints and synergistic drug targets in the sterol biosynthesis pathway of Naegleria fowleri. PLoS Pathogens, 14, e1007245.
Kidane, M. E., Vanderloop, B. H., Zhou, W., Thomas, C. D., Ramos, E., Singha, U., Chaudhuri, M., & Nes, W. D. (2017). Sterol methyltransferase a target for anti-amoeba therapy: Towards transition state analog and suicide substrate drug design. Journal of Lipid Research, 58, 2310–2323.
Debnath, A., Calvet, C. M., Jennings, G., Zhou, W., Aksenov, A., Luth, M. R., Abagyan, R., Nes, W. D., McKerrow, J. H., & Podust, L. M. (2017). CYP51 is an essential drug target for the treatment of primary amoebic meningoencephalitis (PAM). PLOS Neglected Tropical Diseases, 11, e0006104.
Zhou, W., Cross, G. A. M., & Nes, W. D. (2007). Cholesterol import fails to prevent catalyst-based inhibition of ergosterol synthesis and cell proliferation of Trypanosoma brucei. Journal of Lipid Research, 48, 665–673.
Sharfman, M., Bar, M., Schuster, S., Leibman, M., & Avni, A. (2014). Sterol-dependent induction of plant defense responses by a microbe-associated molecular pattern from Trichoderma viride. Plant Physiology, 164, 819–827.
Behmer, S., & Nes, W. (2003). Insect sterol nutrition and physiology: A global overview. Advances in Insect Physiology, 31, 1–72.
Fujimoto, Y., Kimura, M., Takasu, A., Khalifa, F. A. M., & Ikekawa, N. (1984). Mechanism of stigmasterol dealkylation in insect. Tetrahedron Letters, 25, 1501–1504.
Ikekawa, N., Morisaki, M., & Fujimoto, Y. (1993). Sterol metabolism in insects: Dealkylation of phytosterol to cholesterol. Accounts of Chemical Research, 26, 139–146.
Chitwood, D. J., Hutzell, P. A., & Lusby, W. R. (1985). Sterol composition of the corn cyst nematode, heterodera zeae, and corn roots. Journal of Nematology, 17, 64–68.
Chitwood, D. J., & Lusby, W. R. (1991). Sterol composition of the corn root lesion nematode, Pratylenchus agilis, and corn root cultures. Journal of the Helminthological Society of Washington, 581, 43–50.
Ciufo, L. F., Murray, P. A., Thompson, A., Rigden, D. J., & Rees, H. H. (2011). Characterisation of a desmosterol reductase involved in phytosterol dealkylation in the silkworm Bombyx mori. PLoS ONE. https://doi.org/10.1371/journal.pone.0021316
Müller, C., Hank, E., Giera, M., & Bracher, F. (2022). Dehydrocholesterol reductase 24 (DHCR24): Medicinal chemistry, pharmacology and novel therapeutic options. Current Medicinal Chemistry, 29, 4005–4025.
Svoboda, J. A., & Robbins, W. E. (1967). Conversion of beta sitosterol to cholesterol blocked in an insect by hypocholesterolemic agents. Science, 156, 1637–1638.
Mack, Y. S. I., Dehari, M., Morooka, N., & Nagata, S. (2021). Identification and characterization of 24-dehydrocholesterol reductase (DHCR24) in the two-spotted cricket Gryllus bimaculatus. Insects, 12, 782.
Chitwood, D. J., Lusby, W. R., Lozano, R., Thompson, M. J., & Svoboda, J. A. (1984). Sterol metabolism in the nematode Caenorhabditis elegans. Lipids, 19, 500–506.
Waterham, H. R., Koster, J., Romeijn, G. J., Hennekam, R. C. M., Vreken, P., Andersson, H. C., Fitzpatrick, D. R., Kelley, R. I., & Wanders, R. J. A. (2001). Mutations in the 3β-hydroxysterol Δ24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. The American Journal of Human Genetics, 69, 685–694.
Qi, Z., Zhang, Y., Yao, K., Zhang, M., Xu, Y., Zhang, J., Bai, X., & Zu, H. (2021). DHCR24 knockdown lead to Hyperphosphorylation of Tau at Thr181, Thr231, Ser262, Ser396, and Ser422 sites by membrane lipid-raft dependent PP2A Signaling in SH-SY5Y cells. Neurochemical Research, 46, 1627–1640.
Dai, M., Zhu, X.-L., Liu, F., Xu, Q.-Y., Ge, Q.-L., Jiang, S.-H., Yang, X.-M., Li, J., Wang, Y.-H., Wu, Q.-K., et al. (2017). Cholesterol synthetase DHCR24 induced by insulin aggravates cancer invasion and progesterone resistance in endometrial carcinoma. Scientific Reports, 7, 41404.
Kim, Y., Park, Y., Hwang, J., & Kwack, K. (2018). Comparative genomic analysis of the human and nematode Caenorhabditis elegans uncovers potential reproductive genes and disease associations in humans. Physiological Genomics, 50, 1002–1014.
Fujimoto, Y., Ohyama, K., Nomura, K., Hyodo, R., Takahashi, K., Yamada, J., & Morisaki, M. (2000). Biosynthesis of sterols and ecdysteroids in Ajuga hairy roots. Lipids, 35, 279–288.
Merris, M., Wadsworth, W. G., Khamrai, U., Bittman, R., Chitwood, D. J., & Lenard, J. (2003). Sterol effects and sites of sterol accumulation in Caenorhabditis elegans: Developmental requirement for 4 alpha-methyl sterols. Journal of Lipid Research, 44, 172.
He, F. (2011). Synchronization of worm. Bio-protocol, 1, e56.
Sutphin, G. L., & Kaeberlein, M. (2009). Measuring Caenorhabditis elegans life span on solid media. Journal of Visualized Experiments. https://doi.org/10.3791/1152
Shamsuzzama, L. R., Trabelcy, B., Langier Goncalves, I., Gerchman, Y., & Sapir, A. (2020). Metabolic reconfiguration in C. elegans suggests a pathway for widespread sterol auxotrophy in the animal kingdom. Current Biology, 30, 3031-3038 e3037.
Fielenbach, N., & Antebi, A. (2008). C. elegans dauer formation and the molecular basis of plasticity. Genes & Development, 22, 2149–2165.
Bi, Y., Yang, G., Li, H., Zhang, G., & Guo, Z. (2006). Characterization of the chemical composition of lotus plumule oil. Journal of Agricultural and Food Chemistry, 54, 7672–7677.
Moreau, R. A., Whitaker, B. D., & Hicks, K. B. (2002). Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Progress in Lipid Research, 41, 457–500.
Lozano, R., Lusby, W. R., Chitwood, D. J., Thompson, M. J., & Svoboda, J. A. (1985). Inhibition of C28 and C29 phytosterol metabolism by N, N-dimethyldodecanamine in the nematode Caenorhabditis elegans. Lipids, 20, 158–166.
Chitwood, D. J., & Lusby, W. R. (1991). Metabolism of plant sterols by nematodes. Lipids, 26, 619–627.
Lozano, R., Chitwood, D. J., Lusby, W. R., Thompson, M. J., Svoboda, J. A., & Patterson, G. W. (1984). Comparative effects of growth inhibitors on sterol metabolism in the nematode Caenorhabditis elegans. Comparative Biochemistry and Physiology Part C Comparative Pharmacology, 79, 21–26.
Nagase, A., Kuwahara, Y., Tominaga, Y., & Sugawara, R. (1982). Nematicidal activity of akylamine against the pine wood nematode Bursaphelenchus lignicolus. Agricultural and Biological Chemistry, 46, 167–172.
Acknowledgements
We are grateful to the anonymous reviewers for their valuable improvement to this manuscript. This work was supported in part by grants from the National Key R&D Program of Chinas (2021YFA0909600 and 2021YFE0110100), the National Natural Science Foundation of China (32060061 and 32370380), the Key R&D Program of Hainan Province (ZDYF2022XDNY140), the Natural Science Foundation of Hainan Province (322QN250), the Foreign Expert Foundation of Hainan Province (G20230607016E), and the Program of Hainan Provincial Key Laboratory of Tropical Hydrobiotechnology (SWJS202205).
Author information
Authors and Affiliations
Contributions
QG, XC, LZ, WZ and YL conceived and planned the experiments. QG, XC and LZ carried out the experiments. QC and LZ contributed to sample preparation. XC, WZ and YL contributed to the interpretation of the results. YL took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gan, Q., Cui, X., Zhang, L. et al. Control Phytophagous Nematodes By Engineering Phytosterol Dealkylation Caenorhabditis elegans as a Model. Mol Biotechnol (2023). https://doi.org/10.1007/s12033-023-00869-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-023-00869-x