Abstract
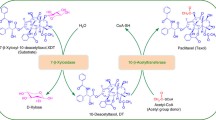
Taxoid 10β-O-acetyltransferase (DBAT) is the key enzyme to produce baccatin III, a key precursor in paclitaxel synthesis, by acetyl group transfer from acetyl-CoA to the C10 hydroxyl of 10-deacetylbaccatin III. In this study, the recombinant DBAT (rDBAT) was immobilized by cross-linked enzyme aggregates (CLEAs). To further optimize the enzyme recovery, single-factor experiment and response surface methodology were applied. 60% ammonium sulfate as precipitant, 0.05% glutaraldehyde as fixing agent, pH 7.0, 2 h as cross-linking time, 30 °C as cross-linking temperature were confirmed to be the optimum conditions to prepare the CLEAs-rDBAT in single-factor experiment. In addition, 62% for ammonium sulfate saturation, 0.15% for glutaraldehyde, and pH 6.75 were confirmed to be the optimum conditions with averagely 73.9% activity recovery in 3 replications, which was consistent with the prediction of response surface methodology. After cross-linking, the optimum temperature of CLEAs-rDBAT rose up to 70 °C and CLEAs-rDBAT could be recycled for three times.




Similar content being viewed by others
References
Hanefeld, U., Cao, L., & Magner, E. (2013). Enzyme immobilisation: Fundamentals and application. Chemical Society Reviews, 42, 6211–6212.
DiCosimo, R., McAuliffe, J., Poulose, A. J., & Bohlmann, G. (2013). Industrial use of immobilized enzymes. Chemical Society Reviews, 42, 6437–6474.
Sheldon, R. A., & Pereira, P. C. (2017). Biocatalysis engineering: The big picture. Chemical Society Reviews, 46, 2678–2691.
Bilal, M., Asgher, M., Iqbal, H. M., Hu, H., & Zhang, X. (2017). Bio-based degradation of emerging endocrine-disrupting and dye-based pollutants using cross-linked enzyme aggregates. Environmental Science and Pollution Research International, 24, 7035–7041.
Chen, L., Hu, Y. D., Li, N., & Zong, M. H. (2012). Cross-linked enzyme aggregates of beta-glucosidase from Prunus domestica seeds. Biotechnology Letters, 34, 1673–1678.
Hu, X., Liu, L., Chen, D., Wang, Y., Zhang, J., & Shao, L. (2017). Co-expression of the recombined alcohol dehydrogenase and glucose dehydrogenase and cross-linked enzyme aggregates stabilization. Bioresource Technology, 224, 531–535.
Guo, B., Kai, G., Gong, Y., **, H., Wang, Y., Miao, Z., et al. (2007). Molecular cloning and heterologous expression of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus x media. Molecular Biology Reports, 34, 89–95.
Loncaric, C., Merriweather, E., & Walker, K. D. (2006). Profiling a Taxol pathway 10beta-acetyltransferase: assessment of the specificity and the production of baccatin III by in vivo acetylation in E. coli. Chemistry & Biology, 13, 309–317.
Walker, K., & Croteau, R. (2000). Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 97, 583–587.
Lin, S. L., Wei, T., Lin, J. F., Guo, L. Q., Wu, G. P., Wei, J. B., et al. (2018). Bio-production of baccatin III, an important precursor of paclitaxel by a cost-effective approach. Molecular Biotechnology, 60, 492–505.
You, L. F., Wei, T., Zheng, Q. W., Lin, J. F., Guo, L. Q., Jiang, B. H., et al. (2018). Activity essential residue analysis of taxoid 10beta-O-acetyl transferase for enzymatic synthesis of baccatin. Applied Biochemistry and Biotechnology. https://doi.org/10.1007/s12010-018-2789-0.
You, L. F., Huang, J. J., Wei, T., Lin, S. L., Jiang, B. H., Guo, L. Q., et al. (2018). Enhanced catalytic activities and modified substrate preferences for taxoid 10beta-O-acetyl transferase mutants by engineering catalytic histidine residues. Biotechnology Letters, 40, 1245–1251.
Zhen, Q., Wang, M., Qi, W., Su, R., & He, Z. (2013). Preparation of β-mannanase CLEAs using macromolecular cross-linkers. Catalysis Science & Technology, 3, 1937–1941.
Ghaffari-Moghaddam, M., Yekke-Ghasemi, Z., Khajeh, M., Rakhshanipour, M., & Yasin, Y. (2014). Application of response surface methodology in enzymatic synthesis: a review. Russian Journal of Bioorganic Chemistry, 40, 252–262.
Schoevaart, R., Wolbers, M. W., Golubovic, M., Ottens, M., Kieboom, A. P., van Rantwijk, F., et al. (2004). Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnology and Bioengineering, 87, 754–762.
You, L. F., Liu, Z. M., Lin, J. F., Guo, L. Q., Huang, X. L., & Yang, H. X. (2014). Molecular cloning of a laccase gene from Ganoderma lucidum and heterologous expression in Pichia pastoris. Journal of Basic Microbiology, 54, S134–S141.
Han, F., Kang, L. Z., Zeng, X. L., Ye, Z. W., Guo, L. Q., & Lin, J. F. (2014). Bioproduction of baccatin III, an advanced precursor of paclitaxol, with transgenic Flammulina velutipes expressing the 10-deacetylbaccatin III-10-O-acetyl transferase gene. Journal of the Science of Food and Agriculture, 94, 2376–2383.
Martins, S. L., Albuquerque, B. F., Nunes, M. A. P., & Ribeiro, M. H. L. (2018). Exploring magnetic and imprinted cross-linked enzyme aggregates of rhamnopyranosidase in microbioreactors. Bioresource Technology, 249, 704–712.
Nadar, S. S., Muley, A. B., Ladole, M. R., & Joshi, P. U. (2016). Macromolecular cross-linked enzyme aggregates (M-CLEAs) of alpha-amylase. International Journal of Biological Macromolecules, 84, 69–78.
Nadar, S. S., & Rathod, V. K. (2016). Magnetic macromolecular cross linked enzyme aggregates (CLEAs) of glucoamylase. Enyzme and Microbial Technology, 83, 78–87.
Unsworth, D. L., Oost, J., Acebal, C., & Koutsopoulos, S. (2007). Hyperthermophilic enzymes—stability, activity and implementation strategies for high temperature applications. FEBS Journal, 274, 4044–4056.
Hormigo, D., Garcia-Hidalgo, J., Acebal, C., de la Mata, I., & Arroyo, M. (2012). Preparation and characterization of cross-linked enzyme aggregates (CLEAs) of recombinant poly-3-hydroxybutyrate depolymerase from Streptomyces exfoliatus. Bioresource Technology, 115, 177–182.
Liu, D. M., Chen, J., & Shi, Y. P. (2018). Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends in Analytical Chemistry, 102, 332–342.
Cui, J. D., Zhang, S., & Sun, L. M. (2012). Cross-linked enzyme aggregates of phenylalanine ammonia lyase: novel biocatalysts for synthesis of l-phenylalanine. Applied Biochemistry and Biotechnology, 167, 835–844.
Cui, J. D., Sun, L. M., & Li, L. L. (2013). A simple technique of preparing stable CLEAs of phenylalanine ammonia lyase using co-aggregation with starch and bovine serum albumin. Applied Biochemistry and Biotechnology, 170, 1827–1837.
Acknowledgements
This work was supported by the Science and Technology Program of Guangdong Province (Grants 2014B050505018, 2014B020205003, 2015A020209121) and the National Natural Science Foundation of China (Grants 31071837, 31572178, 31772373).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
You, LF., Wei, T., Zheng, QW. et al. Optimization of Baccatin III Production by Cross-Linked Enzyme Aggregate of Taxoid 10β-O-Acetyltransferase. Mol Biotechnol 61, 498–505 (2019). https://doi.org/10.1007/s12033-019-00179-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-019-00179-1