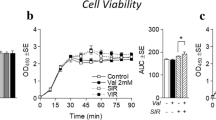
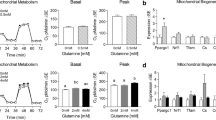
Abstract
Purpose
Branched-chain amino acids (BCAA) have been shown to enhance several cellular signaling pathways including protein synthesis and mitochondrial biogenesis, yet population data demonstrate a correlation between circulating BCAA and severity of insulin resistance which has been hypothesized to be, in part, a byproduct of BCAA inhibition of mitochondrial function. The purpose of this study is to examine the effect of a BCAA mixture on muscle metabolism and related gene expression in vitro.
Methods
C2C12 myotubes were treated with a BCAA mixture containing leucine:isoleucine:valine at a ratio of 2:1:1 at 0.2, 2, or 20 mM (based on leucine content) for 6 days. qRT-PCR was used to measure metabolic gene expression. Oxygen consumption and extracellular acidification were used to assess mitochondrial and glycolytic metabolism, respectively. Mitochondrial content was determined via mitochondrial-specific staining.
Results
Despite significantly elevated mitochondrial staining, 6-day BCAA treatment reduced basal mitochondrial metabolism at a supraphysiological concentration (20 mM) in both insulin sensitive and resistant cells. Peak mitochondrial capacity was also reduced in insulin-resistant (but not insulin sensitive) cells. Conversely, basal glycolytic metabolism was elevated following 20 mM BCAA treatment, regardless of insulin resistance. In addition, insulin-resistant cells treated with 20 mM BCAA exhibited reduced gene expression of Ppargc1a, Cytc, Atp5b, Glut4, and several glycolytic enzymes versus insulin sensitive cells treated with 20 mM BCAA.
Conclusions
Collectively, these findings suggest BCAA at supraphysiologically high levels may negatively alter mitochondrial metabolism, and concurrent insulin resistance may also diminish peak mitochondrial capacity, as well as impede molecular adaptations that support a transition to a glycolytic preference/compensation.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- BCAA:
-
branched-chain amino acid
- BCKDH:
-
branched-chain alpha-keto acid dehydrogenase
- CS:
-
citrate synthase
- ECAR:
-
extracellular acidification rate
- FCCP:
-
carbonyl cyanide p-[trifluoromethoxy]-phenyl-hydrazone
- GLUT4:
-
glucose transporter 4
- IRS-1:
-
insulin receptor substrate 1
- LDHa:
-
lactate dehydrogenase A
- LDHb:
-
lactate dehydrogenase B
- mTOR:
-
mammalian target of rapamycin (mTOR)
- NRF1/2:
-
nuclear respiratory factor 1/2
- OCR:
-
oxygen consumption rate
- PGC-1α:
-
peroxisome proliferator-activated receptor gamma coactivator 1 alpha
- PPARα:
-
peroxisome proliferator-activated receptor alpha
- PPARβ/δ:
-
peroxisome proliferator-activated receptor beta/delta
- PK:
-
pyruvate kinase
- TBP:
-
TATA binding protein
- TFAM:
-
mitochondrial transcription factor A
References
Z. Arany, M. Neinast, Branched chain amino acids in metabolic disease. Curr. Diab. Rep. 18, 76 (2018)
N.P. Gannon, J.K. Schnuck, R.A. Vaughan, BCAA metabolism and insulin sensitivity—dysregulated by metabolic status? Mol. Nutr. Food Res. 62, e1700756 (2018)
M. Holeček, Why are branched-chain amino acids increased in starvation and diabetes? Nutrients 12, 3087 (2020)
C.J. Lynch, S.H. Adams, Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736 (2014)
C.B. Newgard, Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 15, 606–614 (2012)
H.S. Brunetta, C.Q. de Camargo, E.A. Nunes, Does L-leucine supplementation cause any effect on glucose homeostasis in rodent models of glucose intolerance? A systematic review. Amino Acids 50, 1663–1678 (2018)
N.P. Gannon, R.A. Vaughan, Leucine-induced anabolic-catabolism: two sides of the same coin. Amino Acids 48, 321–336 (2015)
L. Zhang, F. Li, Q. Guo, Y. Duan, W. Wang, Y. Zhong, Y. Yang, Y. Yin, Leucine supplementation: a novel strategy for modulating lipid metabolism and energy homeostasis. Nutrients 12, 1299 (2020)
C. Ruocco, A. Segala, A. Valerio, E. Nisoli, Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care 24, 88–95 (2021)
C. Liang, B.J. Curry, P.L. Brown, M.B. Zemel, Leucine modulates mitochondrial biogenesis and SIRT1-AMPK signaling in C2C12 myotubes. J. Nutr. Metab. 2014, 239750 (2014)
J.K. Schnuck, K.L. Sunderland, N.P. Gannon, M.R. Kuennen, R.A. Vaughan, Leucine stimulates PPARbeta/delta-dependent mitochondrial biogenesis and oxidative metabolism with enhanced GLUT4 content and glucose uptake in myotubes. Biochimie 128-129, 1–7 (2016)
X. Sun, M.B. Zemel, Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr. Metab. 6, 26 (2009)
Y. Sato, K.A. Obeng, F. Yoshizawa, Acute oral administration of L-leucine upregulates slow-fiber- and mitochondria-related genes in skeletal muscle of rats. Nutr. Res. 57, 36–44 (2018)
X. Chen, L. **ang, G. Jia, G. Liu, H. Zhao, Z. Huang, Effects of dietary leucine on antioxidant activity and expression of antioxidant and mitochondrial-related genes in longissimus dorsi muscle and liver of piglets. Anim. Sci. J. 90, 990–998 (2019)
X. Chen, L. **ang, G. Jia, G. Liu, H. Zhao, Z. Huang, Leucine regulates slow-twitch muscle fibers expression and mitochondrial function by Sirt1/AMPK signaling in porcine skeletal muscle satellite cells. Anim. Sci. J. 90, 255–263 (2019)
Y. Zhong, L. Zeng, J. Deng, Y. Duan, F. Li, β-hydroxy-β-methylbutyrate (HMB) improves mitochondrial function in myocytes through pathways involving PPARβ/δ and CDK4. Nutrition 60, 217–226 (2018)
G. D’Antona, M. Ragni, A. Cardile, L. Tedesco, M. Dossena, F. Bruttini, F. Caliaro, G. Corsetti, R. Bottinelli, M.O. Carruba, A. Valerio, E. Nisoli, Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 12, 362–372 (2010)
M.A. Johnson, N.P. Gannon, J.K. Schnuck, E.S. Lyon, K.L. Sunderland, R.A. Vaughan, Leucine, Palmitate, or leucine/palmitate cotreatment enhances myotube lipid content and oxidative preference. Lipids 53, 1043–1057 (2018)
M.E. Rivera, E.S. Lyon, M.A. Johnson, R.A. Vaughan, Leucine increases mitochondrial metabolism and lipid content without altering insulin signaling in myotubes. Biochimie 168, 124–133 (2020)
H. Li, M. Xu, J. Lee, C. He, Z. **e, Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 303, E1234–1244 (2012)
J. Jiao, S.F. Han, W. Zhang, J.Y. Xu, X. Tong, X.B. Yin, L.X. Yuan, L.Q. Qin, Chronic leucine supplementation improves lipid metabolism in C57BL/6J mice fed with a high-fat/cholesterol diet. Food Nutr. Res. 60, 31304 (2016)
H. Wu, S. Dridi, Y. Huang, J.I. Baum, Leucine decreases intramyocellular lipid deposition in an mTORC1-independent manner in palmitate-treated C2C12 myotubes. Am. J. Physiol. Endocrinol. Metab. 318, E152–E163 (2020)
R.A. Vaughan, R. Garcia-Smith, N.P. Gannon, M. Bisoffi, K.A. Trujillo, C.A. Conn, Leucine treatment enhances oxidative capacity through complete carbohydrate oxidation and increased mitochondrial density in skeletal muscle cells. Amino Acids 45, 901–911 (2013)
J. Banerjee, A. Bruckbauer, M.B. Zemel, Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism 65, 1679–1691 (2016)
A. Bruckbauer, M.B. Zemel, T. Thorpe, M.R. Akula, A.C. Stuckey, D. Osborne, E.B. Martin, S. Kennel, J.S. Wall, Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. 9, (2012). https://doi.org/10.1186/1743-7075-9-77.
L. **ang, Z. Huang, X. Chen, G. Jia, G. Liu, H. Zhao, Leucine regulates porcine muscle fiber type transformation via adiponectin signaling pathway. Anim. Biotechnol. 11, 1–9 (2021)
S. Jager, C. Handschin, J. Pierre, B.M. Spiegelman, AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1 alpha. Proc. Natl Acad. Sci. USA 104, 12017–12022 (2007)
N. Gleyzer, K. Vercauteren, R.C. Scarpulla, Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell. Biol. 25, 1354–1366 (2005)
R.C. Scarpulla, Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 1813, 1269–1278 (2011)
H.H. Zong, J.M. Ren, L.H. Young, M. Pypaert, J. Mu, M.J. Birnbaum, G.I. Shulman, AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl Acad. Sci. USA 99, 15983–15987 (2002)
M.J. Evans, R.C. Scarpulla, NRF-1—a transactivator of nuclear-encoded respiratory genes in animal-cells. Genes Dev. 4, 1023–1034 (1990)
J.V. Virbasius, R.C. Scarpulla, Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors—a potential link between nuclear and mitochodnrial gene-expression in organelle biogenesis. Proc. Natl Acad. Sci. USA 91, 1309–1313 (1994)
R.C. Scarpulla, Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 286, 81–89 (2002)
J.A. Baur, K.J. Pearson, N.L. Price, H.A. Jamieson, C. Lerin, A. Kalra, V.V. Prabhu, J.S. Allard, G. Lopez-Lluch, K. Lewis, P.J. Pistell, S. Poosala, K.G. Becker, O. Boss, D. Gwinn, M.Y. Wang, S. Ramaswamy, K.W. Fishbein, R.G. Spencer, E.G. Lakatta, D. Le Couteur, R.J. Shaw, P. Navas, P. Puigserver, D.K. Ingram, R. de Cabo, D.A. Sinclair, Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 (2006)
M. Lagouge, C. Argmann, Z. Gerhart-Hines, H. Meziane, C. Lerin, F. Daussin, N. Messadeq, J. Milne, P. Lambert, P. Elliott, B. Geny, M. Laakso, P. Puigserver, J. Auwerx, Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell 127, 1109–1122 (2006)
R. Amat, A. Planavila, S.L. Chen, R. Iglesias, M. Giralt, F. Villarroya, SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma co-activator-1 alpha (PGC-1 alpha) gene in skeletal muscle through the PGC-1 alpha autoregulatory loop and interaction with MyoD. J. Biol. Chem. 284, 21872–21880 (2009)
J. Brenmoehl, A. Hoelflich, Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion 13, 755–761 (2013)
Y. Li, Z. **ong, W. Yan, E. Gao, H. Cheng, G. Wu, Y. Liu, L. Zhang, C. Li, S. Wang, M. Fan, H. Zhao, F. Zhang, L. Tao, Branched chain amino acids exacerbate myocardial ischemia/reperfusion vulnerability via enhancing GCN2/ATF6/PPAR-α pathway-dependent fatty acid oxidation. Theranostics 10, 5623–5640 (2020)
L. Tedesco, F. Rossi, M. Ragni, C. Ruocco, D. Brunetti, M.O. Carruba, Y. Torrente, A. Valerio, E. Nisoli, A Special amino-acid formula tailored to boosting cell respiration prevents mitochondrial dysfunction and oxidative stress caused by doxorubicin in mouse cardiomyocytes. Nutrients 12, 282 (2020)
L. Tedesco, G. Corsetti, C.,M. Ruocco, M. Ragni, F. Rossi, M.O. Carruba, A. Valerio, E. Nisoli, A specific amino acid formula prevents alcoholic liver disease in rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 314, G566–G582 (2018)
N. Abedpoor, F. Taghian, K. Ghaedi, I. Niktab, Z. Safaeinejad, F. Rabiee, S. Tanhaei, M.H. Nasr-Esfahani, PPARγ/Pgc-1α-Fndc5 pathway up-regulation in gastrocnemius and heart muscle of exercised, branched chain amino acid diet fed mice. Nutr. Metab. 15, 59 (2018)
T. Matsumoto, K. Nakamura, H. Matsumoto, R. Sakai, T. Kuwahara, Y. Kadota, Y. Kitaura, J. Sato, Y. Shimomura, Bolus ingestion of individual branched-chain amino acids alters plasma amino acid profiles in young healthy men. Springerplus 3, 35 (2014)
R. Elango, K. Chapman, M. Rafii, R.O. Ball, P.B. Pencharz, Determination of the tolerable upper intake level of leucine in acute dietary studies in young men. Am. J. Clin. Nutr. 96, 759–767 (2012)
E.S. Lyon, M.E. Rivera, M.A. Johnson, K.L. Sunderland, R.A. Vaughan, Actions of chronic physiological 3-hydroxyisobuterate treatment on mitochondrial metabolism and insulin signaling in myotubes. Nutr. Res. 66, 22–31 (2019)
M.E. Rivera, E.S. Lyon, M.A. Johnson, K.L. Sunderland, R.A. Vaughan, Effect of valine on myotube insulin sensitivity and metabolism with and without insulin resistance. Mol. Cell. Biochem. 468, 169–183 (2020)
N. Kumar, C.S. Dey, Metformin enhances insulin signalling in insulin-dependent and-independent pathways in insulin resistant muscle cells. Br. J. Pharm. 137, 329–336 (2002)
N. Kumar, C.S. Dey, Development of insulin resistance and reversal by thiazolidinediones in C2C12 skeletal muscle cells. Biochem. Pharmacol. 65, 249–257 (2003)
M. Rivera, C. Rivera, R. Vaughan, Branched-chain amino acids at supraphysiological but not physiological levels reduce myotube insulin sensitivity. Diabetes Metab. Res. Rev. e3490 (2021). https://doi.org/10.1002/dmrr.3490. Epub ahead of print
H. Crossland, K. Smith, I. Idris, B.E. Phillips, P.J. Atherton, D.J. Wilkinson, Exploring mechanistic links between extracellular BCAA & muscle insulin resistance: an in vitro approach. Am. J. Physiol. Cell. Physiol. 319, C1151–C1157 (2020)
J.J. Petrocelli, M.J. Drummond, PGC-1α-targeted therapeutic approaches to enhance muscle recovery in aging. Int. J. Environ. Res. Public Health 17, 8650 (2020)
Y.B. Chang, K.B. Hong, M.G. Kim, H.J. Suh, K. Jo, Effect of the protein hydrolysate of rice syrup meal on the endurance exercise performance of BALB/c mice. Food Funct. 12, 1338–1348 (2021)
C. Ruocco, M. Ragni, F. Rossi, P. Carullo, V. Ghini, F. Piscitelli, A. Cutignano, E. Manzo, R.M. Ioris, F. Bontems, L. Tedesco, C.M. Greco, A. Pino, I. Severi, D. Liu, R.P. Ceddia, L. Ponzoni, L. Tenori, L. Rizzetto, M. Scholz, K. Tuohy, F. Bifari, V. Di Marzo, C. Luchinat, M.O. Carruba, S. Cinti, I. Decimo, G. Condorelli, R. Coppari, S. Collins, A. Valerio, E. Nisoli, Manipulation of dietary amino acids prevents and reverses obesity in mice through multiple mechanisms that modulate energy homeostasis. Diabetes 69, 2324–2339 (2020)
W.W. French, S. Dridi, S.A. Shouse, H. Wu, A. Hawley, S.O. Lee, X. Gu, J.I. Baum, A high-protein diet reduces weight gain, decreases food intake, decreases liver fat deposition, and improves markers of muscle metabolism in obese Zucker rats. Nutrients 9, 587 (2017)
K.M. Hill, C.G. Stathis, E. Grinfeld, A. Hayes, A.J. McAinch, Co-ingestion of carbohydrate and whey protein isolates enhance PGC-1α mRNA expression: a randomised, single blind, cross over study. J. Int. Soc. Sports Nutr. 10, 8 (2013)
I. Buondonno, F. Sassi, G. Carignano, F. Dutto, C. Ferreri, F.G. Pili, M. Massaia, E. Nisoli, C. Ruocco, P. Porrino, C. Ravetta, C. Riganti, G.C. Isaia, P. D’Amelio, From mitochondria to healthy aging: The role of branched-chain amino acids treatment: MATeR a randomized study. Clin. Nutr. 39, 2080–2091 (2020)
G. Corsetti, E. Pasini, G. D’Antona, E. Nisoli, V. Flati, D. Assanelli, F.S. Dioguardi, R. Bianchi, Morphometric changes induced by amino acid supplementation in skeletal and cardiac muscles of old mice. Am. J. Cardiol. 101, 26E–34E (2008)
D. Brunetti, E. Bottani, A. Segala, S. Marchet, F. Rossi, F. Orlando, M. Malavolta, M.O. Carruba, C. Lamperti, M. Provinciali, E. Nisoli, A. Valerio, Targeting multiple mitochondrial processes by a metabolic modulator prevents sarcopenia and cognitive decline in SAMP8 mice. Front. Pharmacol. 11, 1171 (2020)
Acknowledgements
The authors would like also to thank the Department of Physical Therapy (Congdon School of Health Sciences) for the use of shared lab space and equipment. The mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the authors, contributors, or publisher.
Funding
Support for this work was provided by the Department of Exercise Science within the Congdon School of Health Sciences.
Author information
Authors and Affiliations
Contributions
M.E.R. and C.N.R. conducted experiments and assisted with manuscript preparation. R.A.V. conceived the study, conducted and oversaw experiments, performed all statistical analyses, and oversaw manuscript preparation. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rivera, M.E., Rivera, C.N. & Vaughan, R.A. Excess branched-chain amino acids alter myotube metabolism and substrate preference which is worsened by concurrent insulin resistance. Endocrine 76, 18–28 (2022). https://doi.org/10.1007/s12020-021-02939-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02939-z