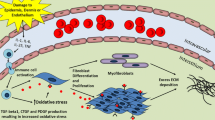
Abstract
Fibroblasts are crucial components of the skin structure. They were traditionally believed to maintain the skin’s structure by producing extracellular matrix and other elements. Recent research illuminated that fibroblasts can respond to external stimuli and exhibit diverse functions, such as the secretion of pro-inflammatory factors, adipogenesis, and antigen presentation, exhibiting remarkable heterogeneity and plasticity. This revelation positions fibroblasts as active contributors to the pathogenesis of skin diseases, challenging the traditional perspective that views fibroblasts solely as structural entities. Based on their diverse functions, fibroblasts can be categorized into six subtypes: pro-inflammatory fibroblasts, myofibroblasts, adipogenic fibroblasts, angiogenic fibroblasts, mesenchymal fibroblasts, and antigen-presenting fibroblasts. Cytokines, metabolism, and epigenetics regulate functional abnormalities in fibroblasts. The dynamic changes fibroblasts exhibit in different diseases and disease states warrant a comprehensive discussion. We focus on dermal fibroblasts’ aberrant manifestations and pivotal roles in inflammatory and autoimmune skin diseases, including psoriasis, vitiligo, lupus erythematosus, scleroderma, and atopic dermatitis, and propose targeting aberrantly activated fibroblasts as a potential therapeutic strategy for inflammatory and autoimmune skin diseases.







Similar content being viewed by others
Data Availability
No datasets were generated or analyzed during the current study.
References
Zhang F, Zhang B, Ding H et al (2023) The oxysterol receptor EBI2 links innate and adaptive immunity to limit IFN response and systemic lupus erythematosus. Adv Sci (Weinh). https://doi.org/10.1002/advs.202207108
Zhao Z, Zhu H, Li Q et al (2022) Skin CD4+ Trm cells distinguish acute cutaneous lupus erythematosus from localized discoid lupus erythematosus/subacute cutaneous lupus erythematosus and other skin diseases. J Autoimmun 128:102811. https://doi.org/10.1016/j.jaut.2022.102811
Relle M, Weinmann-Menke J, Scorletti E et al (2015) Genetics and novel aspects of therapies in systemic lupus erythematosus. Autoimmun Rev 14:1005–1018. https://doi.org/10.1016/j.autrev.2015.07.003
Crow MK (2023) Pathogenesis of systemic lupus erythematosus: risks, mechanisms and therapeutic targets. Ann Rheum Dis 82:999–1014. https://doi.org/10.1136/ard-2022-223741
Uppala R, Tsoi LC, Harms PW et al (2021) “Autoinflammatory psoriasis”-genetics and biology of pustular psoriasis. Cell Mol Immunol 18:307–317. https://doi.org/10.1038/s41423-020-0519-3
Lynch MD, Watt FM (2018) Fibroblast heterogeneity: implications for human disease. J Clin Invest 128:26–35. https://doi.org/10.1172/JCI93555
Buechler MB, Pradhan RN, Krishnamurty AT et al (2021) Cross-tissue organization of the fibroblast lineage. Nature 593:575–579. https://doi.org/10.1038/s41586-021-03549-5
Plikus MV, Wang X, Sinha S et al (2021) Fibroblasts: origins, definitions, and functions in health and disease. Cell 184:3852–3872. https://doi.org/10.1016/j.cell.2021.06.024
Chen SX, Zhang L-J, Gallo RL (2019) Dermal white adipose tissue: a newly recognized layer of skin innate defense. J Invest Dermatol 139:1002–1009. https://doi.org/10.1016/j.jid.2018.12.031
Harper RA, Grove G (1979) Human skin fibroblasts derived from papillary and reticular dermis: differences in growth potential in vitro. Science 204:526–527. https://doi.org/10.1126/science.432659
Philippeos C, Telerman SB, Oulès B et al (2018) Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol 138:811–825. https://doi.org/10.1016/j.jid.2018.01.016
Solé-Boldo L, Raddatz G, Schütz S et al (2020) Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun Biol 3:188. https://doi.org/10.1038/s42003-020-0922-4
Capolupo L, Khven I, Lederer AR et al (2022) Sphingolipids control dermal fibroblast heterogeneity. Science 376:eabh1623. https://doi.org/10.1126/science.abh1623
Cavagnero KJ, Li F, Dokoshi T et al (2024) CXCL12+ dermal fibroblasts promote neutrophil recruitment and host defense by recognition of IL-17. J Exp Med 221:e20231425. https://doi.org/10.1084/jem.20231425
Guo D, Li X, Wang J et al (2024) Single-cell RNA-seq reveals keratinocyte and fibroblast heterogeneity and their crosstalk via epithelial-mesenchymal transition in psoriasis. Cell Death Dis 15:207. https://doi.org/10.1038/s41419-024-06583-z
Deng C-C, Hu Y-F, Zhu D-H et al (2021) Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat Commun 12:3709. https://doi.org/10.1038/s41467-021-24110-y
Zhang L-J, Chen SX, Guerrero-Juarez CF et al (2019) Age-related loss of innate immune antimicrobial function of dermal fat is mediated by transforming growth factor beta. Immunity 50:121-136.e5. https://doi.org/10.1016/j.immuni.2018.11.003
Zhang L-J, Guerrero-Juarez CF, Chen SX et al (2021) Diet-induced obesity promotes infection by impairment of the innate antimicrobial defense function of dermal adipocyte progenitors. Sci Transl Med 13:eabb5280. https://doi.org/10.1126/scitranslmed.abb5280
O’Neill AM, Liggins MC, Seidman JS et al (2022) Antimicrobial production by perifollicular dermal preadipocytes is essential to the pathophysiology of acne. Sci Transl Med 14:eabh1478. https://doi.org/10.1126/scitranslmed.abh1478
Bapat SP, Whitty C, Mowery CT et al (2022) Obesity alters pathology and treatment response in inflammatory disease. Nature 604:337–342. https://doi.org/10.1038/s41586-022-04536-0
Tabib T, Huang M, Morse N et al (2021) Myofibroblast transcriptome indicates SFRP2hi fibroblast progenitors in systemic sclerosis skin. Nat Commun 12:4384. https://doi.org/10.1038/s41467-021-24607-6
Goss G, Rognoni E, Salameti V, Watt FM (2021) Distinct fibroblast lineages give rise to NG2+ pericyte populations in mouse skin development and repair. Front Cell Dev Biol 9:675080. https://doi.org/10.3389/fcell.2021.675080
Jiang D, Christ S, Correa-Gallegos D et al (2020) Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat Commun 11:5653. https://doi.org/10.1038/s41467-020-19425-1
Rosa I, Romano E, Fioretto BS et al (2023) Lymphatic endothelial-to-myofibroblast transition: a potential new mechanism underlying skin fibrosis in systemic sclerosis. Cells-basel 12:2195. https://doi.org/10.3390/cells12172195
Kim S-W, Im G-B, Jeong G-J et al (2021) Delivery of a spheroids-incorporated human dermal fibroblast sheet increases angiogenesis and M2 polarization for wound healing. Biomaterials 275:120954. https://doi.org/10.1016/j.biomaterials.2021.120954
Shams F, Moravvej H, Hosseinzadeh S et al (2022) Overexpression of VEGF in dermal fibroblast cells accelerates the angiogenesis and wound healing function: in vitro and in vivo studies. Sci Rep 12:18529. https://doi.org/10.1038/s41598-022-23304-8
Mauroux A, Joncour P, Brassard-Jollive N et al (2023) Papillary and reticular fibroblasts generate distinct microenvironments that differentially impact angiogenesis. Acta Biomater 168:210–222. https://doi.org/10.1016/j.actbio.2023.06.040
Moreira HR, Cerqueira MT, da Silva LP et al (2023) Pre-selection of fibroblast subsets prompts prevascularization of tissue engineered skin analogues. Biomater Sci-uk 11:5287–5300. https://doi.org/10.1039/d2bm02022j
Pal D, Ghatak S, Singh K et al (2023) Identification of a physiologic vasculogenic fibroblast state to achieve tissue repair. Nat Commun 14:1129. https://doi.org/10.1038/s41467-023-36665-z
Umetsu DT, Pober JS, Jabara HH et al (1985) Human dermal fibroblasts present tetanus toxoid antigen to antigen-specific T cell clones. J Clin Invest 76:254–260. https://doi.org/10.1172/JCI111955
Pereira BI, Devine OP, Vukmanovic-Stejic M et al (2019) Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat Commun 10:2387. https://doi.org/10.1038/s41467-019-10335-5
Shook BA, Wasko RR, Mano O et al (2020) Dermal adipocyte lipolysis and myofibroblast conversion are required for efficient skin repair. Cell Stem Cell 26:880-895.e6. https://doi.org/10.1016/j.stem.2020.03.013
Sun L, Zhang X, Wu S et al (2023) Dynamic interplay between IL-1 and WNT pathways in regulating dermal adipocyte lineage cells during skin development and wound regeneration. Cell Rep 42:112647. https://doi.org/10.1016/j.celrep.2023.112647
Shook BA, Wasko RR, Rivera-Gonzalez GC et al (2018) Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 362:eaar2971. https://doi.org/10.1126/science.aar2971
Plikus MV, Guerrero-Juarez CF, Ito M et al (2017) Regeneration of fat cells from myofibroblasts during wound healing. Science 355:748–752. https://doi.org/10.1126/science.aai8792
Yokota M, Häffner N, Kassier M et al (2021) Staphylococcus aureus impairs dermal fibroblast functions with deleterious effects on wound healing. FASEB Journal: Official Publication of the Federation of American Societies For Experimental Biology 35:e21695
Yin L, Hu Y, Xu J et al (2017) Ultraviolet B inhibits IL-17A/TNF-α-stimulated activation of human dermal fibroblasts by decreasing the expression of IL-17RA and IL-17RC on fibroblasts. Front Immunol. https://doi.org/10.3389/fimmu.2017.00091
Taki Z, Gostjeva E, Thilly W et al (2020) Pathogenic activation of mesenchymal stem cells is induced by the disease microenvironment in systemic sclerosis. Arthritis Rheumatol 72:1361–1374. https://doi.org/10.1002/art.41267
Palumbo-Zerr K, Soare A, Zerr P et al (2017) Composition of TWIST1 dimers regulates fibroblast activation and tissue fibrosis. Ann Rheum Dis 76:244–251. https://doi.org/10.1136/annrheumdis-2015-208470
Fang M, **a J, Wu X et al (2013) Adenosine signaling inhibits CIITA-mediated MHC class II transactivation in lung fibroblast cells. Eur J Immunol 43:2162–2173. https://doi.org/10.1002/eji.201343461
Rosa FM, Fellous M (1988) Regulation of HLA-DR gene by IFN-gamma. Transcriptional and post-transcriptional control J Immunol 140:1660–1664
Lochhead RB, Ordoñez D, Arvikar SL et al (2019) Interferon-gamma production in Lyme arthritis synovial tissue promotes differentiation of fibroblast-like synoviocytes into immune effector cells. Cell Microbiol 21:e12992. https://doi.org/10.1111/cmi.12992
Sugawara E, Kato M, Kudo Y et al (2019) Autophagy promotes citrullination of VIM (vimentin) and its interaction with major histocompatibility complex class II in synovial fibroblasts. Autophagy 16:946–955. https://doi.org/10.1080/15548627.2019.1664144
Zhao X, Psarianos P, Ghoraie LS et al (2019) Metabolic regulation of dermal fibroblasts contributes to skin extracellular matrix homeostasis and fibrosis. Nat Metab 1:147–157. https://doi.org/10.1038/s42255-018-0008-5
Wang P, Wang Q, Yang X et al (2023) Targeting the glycolytic enzyme PGK1 to inhibit the warburg effect: a new strategy for keloid therapy. Plast Reconstr Surg 151:970e–980e. https://doi.org/10.1097/PRS.0000000000010137
Gęgotek A, Bielawska K, Biernacki M et al (2017) Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox Biol 12:733–744. https://doi.org/10.1016/j.redox.2017.04.014
Kruglikov IL, Zhang Z, Scherer PE (2022) Skin aging: dermal adipocytes metabolically reprogram dermal fibroblasts. BioEssays 44:e2100207. https://doi.org/10.1002/bies.202100207
Ma Z, Ding Y, Ding X et al (2023) PDK4 rescues high-glucose-induced senescent fibroblasts and promotes diabetic wound healing through enhancing glycolysis and regulating YAP and JNK pathway. Cell Death Discov 9:424. https://doi.org/10.1038/s41420-023-01725-2
Song J, Zhang H, Wang Z et al (2018) The role of FABP5 in radiation-induced human skin fibrosis. Radiat Res 189:177–186. https://doi.org/10.1667/RR14901.1
Huang L-T, Chou H-C, Chen C-M (2022) Inhibition of FABP4 attenuates hyperoxia-induced lung injury and fibrosis via inhibiting TGF-β signaling in neonatal rats. J Cell Physiol 237:1509–1520. https://doi.org/10.1002/jcp.30622
Zhang Z, Kruglikov I, Zhao S et al (2021) Dermal adipocytes contribute to the metabolic regulation of dermal fibroblasts. Exp Dermatol. https://doi.org/10.1111/exd.14181
Phan QM, Salz L, Kindl SS et al (2023) Lineage commitment of dermal fibroblast progenitors is controlled by Kdm6b-mediated chromatin demethylation. EMBO J 42:e113880. https://doi.org/10.15252/embj.2023113880
He Y, Tsou P-S, Khanna D, Sawalha AH (2018) Methyl-CpG-binding protein 2 mediates antifibrotic effects in scleroderma fibroblasts. Ann Rheum Dis 77:1208–1218. https://doi.org/10.1136/annrheumdis-2018-213022
Dees C, Pötter S, Zhang Y et al (2020) TGF-β-induced epigenetic deregulation of SOCS3 facilitates STAT3 signaling to promote fibrosis. J Clin Invest 130:2347–2363. https://doi.org/10.1172/JCI122462
Guo J-R, Yin L, Chen Y-Q et al (2018) Autologous blood transfusion augments impaired wound healing in diabetic mice by enhancing lncRNA H19 expression via the HIF-1α signaling pathway. Cell Commun Signal 16:84. https://doi.org/10.1186/s12964-018-0290-6
Gesumaria L, Matsui MS, Kluz T, Costa M (2015) Solar-simulated ultraviolet radiation induces histone 3 methylation changes in the gene promoters of matrix metalloproteinases 1 and 3 in primary human dermal fibroblasts. Exp Dermatol 24:384–385. https://doi.org/10.1111/exd.12675
Kim M-K, Shin HS, Shin MH et al (2023) Dual role of enhancer of zeste homolog 2 in the regulation of ultraviolet radiation-induced matrix metalloproteinase-1 and type I procollagen expression in human dermal fibroblasts. Matrix Biol 119:112–124. https://doi.org/10.1016/j.matbio.2023.04.001
Tsitsipatis D, Martindale JL, Mazan-Mamczarz K et al (2023) Transcriptomes of human primary skin fibroblasts of healthy individuals reveal age-associated mRNAs and long noncoding RNAs. Aging Cell 22:e13915. https://doi.org/10.1111/acel.13915
Lee K-W, Yeo S-Y, Gong J-R et al (2022) PRRX1 is a master transcription factor of stromal fibroblasts for myofibroblastic lineage progression. Nat Commun 13:2793. https://doi.org/10.1038/s41467-022-30484-4
Wu R, Zeng J, Yuan J et al (2018) MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J Clin Invest 128:2551–2568. https://doi.org/10.1172/JCI97426
Blauvelt A, Chiricozzi A (2018) The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 55:379–390. https://doi.org/10.1007/s12016-018-8702-3
Yang W, He R, Qu H et al (2023) FXYD3 enhances IL-17A signaling to promote psoriasis by competitively binding TRAF3 in keratinocytes. Cell Mol Immunol 20:292–304. https://doi.org/10.1038/s41423-023-00973-7
Ni X, Xu Y, Wang W et al (2022) IL-17D-induced inhibition of DDX5 expression in keratinocytes amplifies IL-36R-mediated skin inflammation. Nat Immunol 23:1577–1587. https://doi.org/10.1038/s41590-022-01339-3
Wang Z, Sun Y, Lou F et al (2022) Targeting the transcription factor HES1 by L-menthol restores protein phosphatase 6 in keratinocytes in models of psoriasis. Nat Commun 13:7815. https://doi.org/10.1038/s41467-022-35565-y
Zhang M, Li N, Cai R et al (2021) Rosmarinic acid protects mice from imiquimod induced psoriasis-like skin lesions by inhibiting the IL-23/Th17 axis via regulating Jak2/Stat3 signaling pathway. Phytother Res 35:4526–4537. https://doi.org/10.1002/ptr.7155
van der Fits L, Mourits S, Voerman JSA et al (2009) Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 182:5836–5845. https://doi.org/10.4049/jimmunol.0802999
Werner S, Krieg T, Smola H (2007) Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol 127:998–1008. https://doi.org/10.1038/sj.jid.5700786
Cai X, Han M, Lou F et al (2023) Tenascin C+ papillary fibroblasts facilitate neuro-immune interaction in a mouse model of psoriasis. Nat Commun 14:2004. https://doi.org/10.1038/s41467-023-37798-x
Angiolilli C, Leijten EFA, Bekker CPJ et al (2022) ZFP36 family members regulate the proinflammatory features of psoriatic dermal fibroblasts. J Invest Dermatol 142:402–413. https://doi.org/10.1016/j.jid.2021.06.030
Dimon-Gadal S, Gerbaud P, Thérond P et al (2000) Increased oxidative damage to fibroblasts in skin with and without lesions in psoriasis. J Invest Dermatol 114:984–989. https://doi.org/10.1046/j.1523-1747.2000.00962.x
Grivas A, Grigoriou M, Malissovas N et al (2022) Combined - whole blood and skin fibroblasts- transcriptomic analysis in psoriatic arthritis reveals molecular signatures of activity, resistance and early response to treatment. Front Immunol 13:964274. https://doi.org/10.3389/fimmu.2022.964274
Kim J, He Y, Tormen S et al (2022) The p300/CBP inhibitor A485 normalizes psoriatic fibroblast gene expression in vitro and reduces psoriasis-like skin inflammation in vivo. J Invest Dermatol 1523-1747. https://doi.org/10.1016/j.jid.2022.09.004
Gao Y, Yao X, Zhai Y et al (2021) Single cell transcriptional zonation of human psoriasis skin identifies an alternative immunoregulatory axis conducted by skin resident cells. Cell Death Dis 12:450. https://doi.org/10.1038/s41419-021-03724-6
Gęgotek A, Domingues P, Wroński A, Skrzydlewska E (2020) Changes in proteome of fibroblasts isolated from psoriatic skin lesions. Int J Mol Sci 21:5363. https://doi.org/10.3390/ijms21155363
Łuczaj W, Wroński A, Domingues P et al (2020) Lipidomic analysis reveals specific differences between fibroblast and keratinocyte ceramide profile of patients with psoriasis vulgaris. Molecules 25:630. https://doi.org/10.3390/molecules25030630
Ommen P, Stjernholm T, Kragstrup T et al (2016) The role of leptin in psoriasis comprises a proinflammatory response by the dermal fibroblast. Br J Dermatol 174:187–190. https://doi.org/10.1111/bjd.13969
Bertesi M, Fantini S, Alecci C et al (2020) Promoter methylation leads to decreased ZFP36 expression and deregulated NLRP3 inflammasome activation in psoriatic Fibroblasts. Front Med (Lausanne) 7:579383. https://doi.org/10.3389/fmed.2020.579383
Werner S (1998) Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev 9:153–165. https://doi.org/10.1016/s1359-6101(98)00010-0
Kovacs D, Falchi M, Cardinali G et al (2005) Immunohistochemical analysis of keratinocyte growth factor and fibroblast growth factor 10 expression in psoriasis. Exp Dermatol 14:130–137. https://doi.org/10.1111/j.0906-6705.2005.00261.x
Gubán B, Vas K, Balog Z et al (2016) Abnormal regulation of fibronectin production by fibroblasts in psoriasis. Br J Dermatol 174:533–541. https://doi.org/10.1111/bjd.14219
Iwata H, Haga N, Ujiie H (2021) Possible role of epiregulin from dermal fibroblasts in the keratinocyte hyperproliferation of psoriasis. J Dermatol 48:1433–1438. https://doi.org/10.1111/1346-8138.16003
Xu X, Prens E, Florencia E et al (2021) Interleukin-17A drives IL-19 and IL-24 expression in skin stromal cells regulating keratinocyte proliferation. Front Immunol 12:719562
Quan C, Cho MK, Shao Y et al (2015) Dermal fibroblast expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal keratinocyte proliferation in normal and diseased skin. Protein Cell 6:890–903. https://doi.org/10.1007/s13238-015-0198-5
Becatti M, Barygina V, Mannucci A et al (2018) Sirt1 protects against oxidative stress-induced apoptosis in fibroblasts from psoriatic patients: a new insight into the pathogenetic mechanisms of psoriasis. Int J Mol Sci 19:1572. https://doi.org/10.3390/ijms19061572
Barygina V, Becatti M, Prignano F et al (2019) Fibroblasts to keratinocytes redox signaling: the possible role of ROS in psoriatic plaque formation. Antioxidants (Basel) 8:566. https://doi.org/10.3390/antiox8110566
Saiag P, Coulomb B, Lebreton C et al (1985) Psoriatic fibroblasts induce hyperproliferation of normal keratinocytes in a skin equivalent model in vitro. Science 230:669–672. https://doi.org/10.1126/science.2413549
Ma F, Plazyo O, Billi AC et al (2023) Single cell and spatial sequencing define processes by which keratinocytes and fibroblasts amplify inflammatory responses in psoriasis. Nat Commun 14:3455. https://doi.org/10.1038/s41467-023-39020-4
Li Z, Cao T, Li Q et al (2023) Cross-disease characterization of fibroblast heterogeneities and their pathogenic roles in skin inflammation. Clin Immunol 255:109742. https://doi.org/10.1016/j.clim.2023.109742
Schirmer C, Klein C, von Bergen M et al (2010) Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells. Blood 116:1715–1725. https://doi.org/10.1182/blood-2010-01-263509
Noack M, Ndongo-Thiam ND, Miossec P (2016) Role of podoplanin in the high interleukin-17A secretion resulting from interactions between activated lymphocytes and psoriatic skin-derived mesenchymal cells. Clin Exp Immunol. https://doi.org/10.1111/cei.12830
Glowacka E, Lewkowicz P, Rotsztejn H, Zalewska A (2010) IL-8, IL-12 and IL-10 cytokines generation by neutrophils, fibroblasts and neutrophils- fibroblasts interaction in psoriasis. Adv Med Sci 55:254–260. https://doi.org/10.2478/v10039-010-0037-0
Rinaldi AO, Korsfeldt A, Ward S et al (2021) Electrical impedance spectroscopy for the characterization of skin barrier in atopic dermatitis. Allergy 76:3066–3079. https://doi.org/10.1111/all.14842
van den Bogaard EH, Elias PM, Goleva E et al (2023) Targeting Skin barrier function in atopic dermatitis. J Allergy Clin Immunol Pract 11:1335–1346. https://doi.org/10.1016/j.jaip.2023.02.005
Park Y-D, Jang H-S, Kim S-Y et al (2006) Two-dimensional electrophoretic profiling of atopic dermatitis in primary cultured keratinocytes from patients. Proteomics 6:1362–1370. https://doi.org/10.1002/pmic.200500277
Leyva-Castillo JM, Sun L, Wu S-Y et al (2022) Single cell transcriptome profile of mouse skin undergoing antigen driven allergic inflammation recapitulates findings in atopic dermatitis skin lesions. J Allergy Clin Immunol 150:373–384. https://doi.org/10.1016/j.jaci.2022.03.002
Park Y-D, Lyou Y-J, Lee K-J et al (2006) Towards profiling the gene expression of fibroblasts from atopic dermatitis patients: human 8K complementary DNA microarray. Clin Exp Allergy 36:649–657. https://doi.org/10.1111/j.1365-2222.2006.02480.x
Villagomez MT, Bae S-J, Ogawa I et al (2004) Tumour necrosis factor-alpha but not interferon-gamma is the main inducer of inducible protein-10 in skin fibroblasts from patients with atopic dermatitis. Br J Dermatol 150:910–916. https://doi.org/10.1111/j.1365-2133.2004.05937.x
Ghosh D, Bernstein JA, Hershey GKK et al (2018) Leveraging multilayered “omics” data for atopic dermatitis: a road map to precision medicine. Front Immunol. https://doi.org/10.3389/fimmu.2018.02727
Zhang B, Roesner LM, Traidl S et al (2023) Single-cell profiles reveal distinctive immune response in atopic dermatitis in contrast to psoriasis. Allergy 78:439–453. https://doi.org/10.1111/all.15486
He H, Suryawanshi H, Morozov P et al (2020) Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J Allergy Clin Immunol 145:1615–1628. https://doi.org/10.1016/j.jaci.2020.01.042
Mitamura Y, Reiger M, Kim J et al (2023) Spatial transcriptomics combined with single-cell RNA-sequencing unravels the complex inflammatory cell network in atopic dermatitis. Allergy 78:2215–2231. https://doi.org/10.1111/all.15781
Berroth A, Kühnl J, Kurschat N et al (2013) Role of fibroblasts in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol 131:1547–1554. https://doi.org/10.1016/j.jaci.2013.02.029
Löwa A, Graff P, Kaessmeyer S, Hedtrich S (2020) Fibroblasts from atopic dermatitis patients trigger inflammatory processes and hyperproliferation in human skin equivalents. J Eur Acad Dermatol Venereol 34:e262–e265. https://doi.org/10.1111/jdv.16240
Hou T, Sun X, Zhu J et al (2020) IL-37 ameliorating allergic inflammation in atopic dermatitis through regulating microbiota and AMPK-mTOR signaling pathway-modulated autophagy mechanism. Front Immunol 11:752. https://doi.org/10.3389/fimmu.2020.00752
Jiao D, Wong C-K, Qiu H-N et al (2016) NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell Mol Immunol 13:535–550. https://doi.org/10.1038/cmi.2015.77
Wong C-K, Leung KM-L, Qiu H-N et al (2012) Activation of eosinophils interacting with dermal fibroblasts by pruritogenic cytokine IL-31 and alarmin IL-33: implications in atopic dermatitis. PLoS ONE 7:e29815. https://doi.org/10.1371/journal.pone.0029815
Gahr N, Fölster-Holst R, Weichenthal M et al (2011) Dermal fibroblasts from acute inflamed atopic dermatitis lesions display increased eotaxin/CCL11 responsiveness to interleukin-4 stimulation. Br J Dermatol 164:586–592. https://doi.org/10.1111/j.1365-2133.2010.10112.x
Ko KI, Merlet JJ, DerGarabedian BP et al (2022) NF-κB perturbation reveals unique immunomodulatory functions in Prx1+ fibroblasts that promote development of atopic dermatitis. Sci Transl Med 14:eabj0324
Nunomura S, Ejiri N, Kitajima M et al (2019) Establishment of a mouse model of atopic dermatitis by deleting Ikk2 in dermal fibroblasts. J Invest Dermatol 139:1274–1283. https://doi.org/10.1016/j.jid.2018.10.047
Wang Y, Li S, Li C (2021) Clinical features, immunopathogenesis, and therapeutic strategies in vitiligo. Clin Rev Allergy Immunol 61:299–323. https://doi.org/10.1007/s12016-021-08868-z
** R, Zhou M, Lin F et al (2023) Pathogenic Th2 cytokine profile skewing by IFN-γ-responding vitiligo fibroblasts via CCL2/CCL8. Cells 12:217. https://doi.org/10.3390/cells12020217
Rani S, Bhardwaj S, Srivastava N et al (2017) Senescence in the lesional fibroblasts of non-segmental vitiligo patients. Arch Dermatol Res 309:123–132. https://doi.org/10.1007/s00403-016-1713-0
Kovacs D, Bastonini E, Ottaviani M et al (2018) Vitiligo skin: exploring the dermal compartment. J Invest Dermatol 138:394–404. https://doi.org/10.1016/j.jid.2017.06.033
Rani S, Chauhan R, Parsad D, Kumar R (2018) Effect of Dickkopf1 on the senescence of melanocytes: in vitro study. Arch Dermatol Res 310:343–350. https://doi.org/10.1007/s00403-018-1820-1
Yamaguchi Y, Passeron T, Watabe H et al (2007) The effects of dickkopf 1 on gene expression and Wnt signaling by melanocytes: mechanisms underlying its suppression of melanocyte function and proliferation. J Invest Dermatol 127:1217–1225. https://doi.org/10.1038/sj.jid.5700629
Abdou AG, Maraee AH, Shoeib MAE-M, Elbana R (2012) Immunolocalization of tenascin-C in vitiligo. Appl Immunohistochem Mol Morphol 20:501–511. https://doi.org/10.1097/PAI.0b013e318246c793
Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK (1997) Tenascin is overexpressed in vitiligo lesional skin and inhibits melanocyte adhesion. Br J Dermatol 137:171–178. https://doi.org/10.1046/j.1365-2133.1997.18011894.x
Chen J, Zhang L, Li Y et al (2024) The effect of abnormal secretion of DKK1 by fibroblasts on melanocytes function in vitiligo. J Eur Acad Dermatol Venereol. https://doi.org/10.1111/jdv.19842
Esmat SM, Hadidi HHE, Hegazy RA et al (2018) Increased tenascin C and DKK1 in vitiligo: possible role of fibroblasts in acral and non-acral disease. Arch Dermatol Res 310:425–430. https://doi.org/10.1007/s00403-018-1830-z
Yamaguchi Y, Itami S, Watabe H et al (2004) Mesenchymal-epithelial interactions in the skin: increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J Cell Biol 165:275–285. https://doi.org/10.1083/jcb.200311122
Purpura V, Persechino F, Belleudi F et al (2014) Decreased expression of KGF/FGF7 and its receptor in pathological hypopigmentation. J Cell Mol Med 18:2553–2557. https://doi.org/10.1111/jcmm.12411
Rani S, Kumari U, Bhardwaj S et al (2019) Decreased expression of neuregulin1 in the lesional skin of vitiligo patients. Int J Dermatol 58:242–249. https://doi.org/10.1111/ijd.14161
Seif El Nasr H, Shaker OG, Fawzi MMT, El-Hanafi G (2013) Basic fibroblast growth factor and tumour necrosis factor alpha in vitiligo and other hypopigmented disorders: suggestive possible therapeutic targets. J Eur Acad Dermatol Venereol 27:103–108. https://doi.org/10.1111/j.1468-3083.2011.04368.x
Xu Z, Chen D, Hu Y et al (2022) Anatomically distinct fibroblast subsets determine skin autoimmune patterns. Nature 601:118–124
** R, Xu H, Zhou M et al (2023) EGR1 mediated reduction of fibroblast secreted-TGF-β1 exacerbated CD8+ T cell inflammation and migration in vitiligo. Inflammation. https://doi.org/10.1007/s10753-023-01922-2
Zou P, **ao Y, Deng Q et al (2022) Occludin promotes adhesion of CD8+ T cells and melanocytes in vitiligo via the HIF-1α signaling pathway. Oxid Med Cell Longev. https://doi.org/10.1155/2022/6732972
Gur C, Wang S-Y, Sheban F et al (2022) LGR5 expressing skin fibroblasts define a major cellular hub perturbed in scleroderma. Cell 185:1373-1388.e20. https://doi.org/10.1016/j.cell.2022.03.011
Zhu H, Luo H, Skaug B et al (2023) Fibroblast subpopulations in systemic sclerosis: functional implications of individual subpopulations and correlations with clinical features. J Invest Dermatol. https://doi.org/10.1016/j.jid.2023.09.288
Yasuoka H, Ihn H, Medsger TA et al (2003) A novel protein highly expressed in testis is overexpressed in systemic sclerosis fibroblasts and targeted by autoantibodies. J Immunol 171:6883–6890. https://doi.org/10.4049/jimmunol.171.12.6883
Zhou X, Tan FK, **ong M et al (2001) Systemic sclerosis (scleroderma): specific autoantigen genes are selectively overexpressed in scleroderma fibroblasts. J Immunol 167:7126–7133. https://doi.org/10.4049/jimmunol.167.12.7126
Werner G, Sanyal A, Mirizio E et al (2023) Single-cell transcriptome analysis identifies subclusters with inflammatory fibroblast responses in localized scleroderma. Int J Mol Sci 24:9796. https://doi.org/10.3390/ijms24129796
**ao R, Liu F-Y, Luo J-Y et al (2006) Effect of small interfering RNA on the expression of connective tissue growth factor and type I and III collagen in skin fibroblasts of patients with systemic sclerosis. Br J Dermatol 155:1145–1153. https://doi.org/10.1111/j.1365-2133.2006.07438.x
Rius Rigau A, Li Y-N, Matei A-E et al (2024) Characterization of vascular niche in systemic sclerosis by spatial proteomics. Circ Res 134:875–891. https://doi.org/10.1161/CIRCRESAHA.123.323299
Ma F, Tsou P-S, Gharaee-Kermani M et al (2024) Systems-based identification of the Hippo pathway for promoting fibrotic mesenchymal differentiation in systemic sclerosis. Nat Commun 15:210. https://doi.org/10.1038/s41467-023-44645-6
Jussila AR, Zhang B, Caves E et al (2022) Skin fibrosis and recovery is dependent on Wnt activation via DPP4. J Invest Dermatol 142:1597-1606.e9. https://doi.org/10.1016/j.jid.2021.10.025
Saigusa R, Asano Y, Nakamura K et al (2017) Systemic sclerosis dermal fibroblasts suppress Th1 cytokine production via galectin-9 overproduction due to Fli1 deficiency. J Invest Dermatol 137:1850–1859. https://doi.org/10.1016/j.jid.2017.04.035
Zheng M, Hu Z, Mei X et al (2022) Single-cell sequencing shows cellular heterogeneity of cutaneous lesions in lupus erythematosus. Nat Commun 13:7489
Zheng M, Hu Z, Zhou W et al (2022) Single-cell transcriptome reveals immunopathological cell composition of skin lesions in subacute cutaneous lupus erythematosus. Clin Immunol (Orlando, Fla) 245:109172
Shoffner-Beck SK, Abernathy-Close L, Lazar S et al (2024) Lupus dermal fibroblasts are proinflammatory and exhibit a profibrotic phenotype in scarring skin disease. JCI Insight. https://doi.org/10.1172/jci.insight.173437
Frangou E, Chrysanthopoulou A, Mitsios A et al (2019) REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann Rheum Dis 78:238–248
Zeng Q, Wang S, Li M et al (2023) Spleen fibroblastic reticular cell-derived acetylcholine promotes lipid metabolism to drive autoreactive B cell responses. Cell Metab 35:837-854.e8. https://doi.org/10.1016/j.cmet.2023.03.010
Furukawa F, Lyon MB, Norris DA (1989) Susceptible cytotoxicity to ultraviolet B light in fibroblasts and keratinocytes cultured from autoimmune-prone MRL/Mp-lpr/lpr mice. Clin Immunol Immunopathol 52:460–472. https://doi.org/10.1016/0090-1229(89)90160-8
Krueger JG, Wharton KA, Schlitt T et al (2019) IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol 144:750–763. https://doi.org/10.1016/j.jaci.2019.04.029
Reich K, Sullivan J, Arenberger P et al (2021) Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5-year results from the randomized placebo-controlled TRANSFIGURE study. Br J Dermatol. https://doi.org/10.1111/bjd.19262
Paller AS, Siegfried EC, Thaçi D et al (2020) Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol 83:1282–1293. https://doi.org/10.1016/j.jaad.2020.06.054
Bieber T, Simpson EL, Silverberg JI et al (2021) Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med 384:1101–1112. https://doi.org/10.1056/NEJMoa2019380
Blair HA, Duggan ST (2018) Belimumab: a review in systemic lupus erythematosus. Drugs 78:355–366. https://doi.org/10.1007/s40265-018-0872-z
Rovin BH, Furie R, Teng YKO et al (2022) A secondary analysis of the Belimumab International Study in Lupus Nephritis trial examined effects of belimumab on kidney outcomes and preservation of kidney function in patients with lupus nephritis. Kidney Int 101:403–413. https://doi.org/10.1016/j.kint.2021.08.027
Schett G, Mackensen A, Mougiakakos D (2023) CAR T-cell therapy in autoimmune diseases. Lancet S0140–6736(23):01126–01131. https://doi.org/10.1016/S0140-6736(23)01126-1
Mackensen A, Müller F, Mougiakakos D et al (2022) Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med 28:2124–2132. https://doi.org/10.1038/s41591-022-02017-5
Friščić J, Böttcher M, Reinwald C et al (2021) The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity 54:1002-1021.e10. https://doi.org/10.1016/j.immuni.2021.03.003
Wu J, Feng Z, Chen L et al (2022) TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat Commun 13:676. https://doi.org/10.1038/s41467-021-27948-4
Wang L-CS, Lo A, Scholler J et al (2014) Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res 2:154–166. https://doi.org/10.1158/2326-6066.CIR-13-0027
Hiltbrunner S, Britschgi C, Schuberth P et al (2021) Local delivery of CAR T cells targeting fibroblast activation protein is safe in patients with pleural mesothelioma: first report of FAPME, a phase I clinical trial. Ann Oncol 32:120–121. https://doi.org/10.1016/j.annonc.2020.10.474
Sakemura R, Hefazi M, Siegler EL et al (2022) Targeting cancer-associated fibroblasts in the bone marrow prevents resistance to CART-cell therapy in multiple myeloma. Blood 139:3708–3721. https://doi.org/10.1182/blood.2021012811
Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA (2006) Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest 116:1955–1962. https://doi.org/10.1172/JCI26532
Duperret EK, Trautz A, Ammons D et al (2018) Alteration of the tumor stroma using a consensus DNA vaccine targeting fibroblast activation protein (FAP) synergizes with antitumor vaccine therapy in mice. Clin Cancer Res 24:1190–1201. https://doi.org/10.1158/1078-0432.CCR-17-2033
Dorst DN, van Caam APM, Vitters EL et al (2021) Fibroblast activation protein targeted photodynamic therapy selectively kills activated skin fibroblasts from systemic sclerosis patients and prevents tissue contraction. Int J Mol Sci 22:12681. https://doi.org/10.3390/ijms222312681
Viale DL, Cafferata EG, Gould D et al (2013) Therapeutic improvement of a stroma-targeted CRAd by incorporating motives responsive to the melanoma microenvironment. J Invest Dermatol 133:2576–2584. https://doi.org/10.1038/jid.2013.191
Francis L, McCluskey D, Ganier C et al (2024) Single-cell analysis of psoriasis resolution demonstrates an inflammatory fibroblast state targeted by IL-23 blockade. Nat Commun 15:913. https://doi.org/10.1038/s41467-024-44994-w
Chakraborty D, Šumová B, Mallano T et al (2017) Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun 8:1130. https://doi.org/10.1038/s41467-017-01236-6
Khanna D, Padilla C, Tsoi LC et al (2022) Tofacitinib blocks IFN-regulated biomarker genes in skin fibroblasts and keratinocytes in a systemic sclerosis trial. JCI Insight 7:e159566. https://doi.org/10.1172/jci.insight.159566
Kitanaga Y, Imamura E, Nakahara Y et al (2020) In vitro pharmacological effects of peficitinib on lymphocyte activation: a potential treatment for systemic sclerosis with JAK inhibitors. Rheumatology (Oxford) 59:1957–1968. https://doi.org/10.1093/rheumatology/kez526
Shi-Wen X, Racanelli M, Ali A et al (2021) Verteporfin inhibits the persistent fibrotic phenotype of lesional scleroderma dermal fibroblasts. J Cell Commun Signal 15:71–80. https://doi.org/10.1007/s12079-020-00596-x
Denton CP, Ong VH, Xu S et al (2018) Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Ann Rheum Dis 77:1362–1371. https://doi.org/10.1136/annrheumdis-2018-213031
Thiolat A, Semerano L, Pers YM et al (2014) Interleukin-6 receptor blockade enhances CD39+ regulatory T cell development in rheumatoid arthritis and in experimental arthritis. Arthritis Rheumatol 66:273–283. https://doi.org/10.1002/art.38246
Rivellese F, Surace AEA, Goldmann K et al (2022) Rituximab versus tocilizumab in rheumatoid arthritis: synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat Med 28:1256–1268. https://doi.org/10.1038/s41591-022-01789-0
Biffi G, Oni TE, Spielman B et al (2019) IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 9:282–301. https://doi.org/10.1158/2159-8290.CD-18-0710
Widjaja AA, Chothani S, Viswanathan S et al (2022) IL11 stimulates IL33 expression and proinflammatory fibroblast activation across tissues. Int J Mol Sci 23:8900. https://doi.org/10.3390/ijms23168900
McGee HM, Schmidt BA, Booth CJ et al (2013) IL-22 promotes fibroblast-mediated wound repair in the skin. J Invest Dermatol 133:1321–1329. https://doi.org/10.1038/jid.2012.463
Zhou X, Hu H, Huynh M-LN et al (2007) Mechanisms of tissue inhibitor of metalloproteinase 1 augmentation by IL-13 on TGF-beta 1-stimulated primary human fibroblasts. J Allergy Clin Immunol 119:1388–1397. https://doi.org/10.1016/j.jaci.2007.02.011
Wu X, Ming B, Wu T et al (2022) IL-33/ST2 axis contributes to the dermal fibrosis of systemic sclerosis via promoting fibroblasts activation. J Dermatol Sci 107:95–104. https://doi.org/10.1016/j.jdermsci.2022.07.009
**ng X, Li A, Tan H, Zhou Y (2020) IFN-γ+ IL-17+ Th17 cells regulate fibrosis through secreting IL-21 in systemic scleroderma. J Cell Mol Med 24:13600–13608. https://doi.org/10.1111/jcmm.15266
Kuzumi A, Yoshizaki A, Matsuda KM et al (2021) Interleukin-31 promotes fibrosis and T helper 2 polarization in systemic sclerosis. Nat Commun 12:5947. https://doi.org/10.1038/s41467-021-26099-w
Acknowledgements
Figures were created using https://BioRender.com.
Funding
This work was supported by the National Key R&D Program of China (2022YFC3601800), the National Natural Science Foundation of China (No. 82030097 and No. 32141004), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2022-RC310-04), and the CAMS Innovation Fund for Medical Sciences (CIFMS) No.2021-I2M-1-059.
Author information
Authors and Affiliations
Contributions
**aoyun Chen wrote and revised the manuscript. Yutong Wu revised the manuscript. SuJie Jia and Ming Zhao provided crucial advice and revised the manuscript. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Wu, Y., Jia, S. et al. Fibroblast: A Novel Target for Autoimmune and Inflammatory Skin Diseases Therapeutics. Clinic Rev Allerg Immunol (2024). https://doi.org/10.1007/s12016-024-08997-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12016-024-08997-1