Abstract
The differentiation, migration, and proliferation of skin fibroblasts are identified as key factors in cutaneous wound healing. Adipose-derived mesenchymal stem cells (ADMSCs) and their exosomes (ADMSC-Exos) have been considered as potential therapeutic tools for tissue regeneration; however, the underlying mechanisms on cutaneous wound healing are still not well understood. In this study, we successfully obtained ADMSC-Exos and found ADMSC-Exos significantly promoted the migration and proliferation of fibroblasts in a dose-dependent manner in vitro. The expression levels of COL-I and COL-III in fibroblasts treated with ADMSC-Exos were significantly increased, while the expression level of α-SMA was decreased. In addition, the enhanced protein expression of WNT2b and β-catenin confirmed the activation of the WNT/β-catenin signaling pathway and the WNT/β-catenin inhibitor (XAV939) reversed the promoting effect of ADMSC-Exos on wound healing and the β-catenin expression. Taken together, our study partially elucidates the mechanism of ADMSC-Exos in wound healing, illustrating the potential of ADMSC-Exos as a new therapeutic approach to promote skin wound healing.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
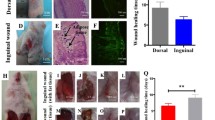
Cutaneous wound healing is a complex and continuous process that includes haemostatic, inflammatory, proliferative, and remodelling stages. Various factors, such as high wound tension, infection, radiation damage, and metabolic disease, lead to prolonged wound healing time, causing physical or mental pain and imposing a severe treatment burden on patients [1, 39]; collagen deposition early in wound healing contributes to wound healing, but later collagen deposition leads to the formation of unsightly scars and organ dysfunction [40]. As for our results, the high concentration of ADMSC-Exos reduced collagen formation and increased the migration and proliferation of fibroblasts, which may be one of the reasons why ADMSC-Exos accelerated wound healing and weakened scar formation. Unfortunately, the specific mechanism is still unclear and needs further study. We hypothesized that these effects were controlled by the complex contents of exosomes, which was shown by our concentration gradient assay.
For another, α-SMA is the marker of fibroblast differentiation into myofibroblast [41], which is closely related to the mechanical tension of wound surface, and excessive α-SMA expression is also the cause of promoting scar formation. Regarding the decreased expression of α-SMA in our research, we predicted that the application of ADMSC-Exos to the wound increased the migration rate of fibroblasts from the periphery to the centre of the wound surface, thus reducing the surface tension. Meanwhile, we speculated that ADMSC-Exos may have been able to inhibit the conversion of fibroblasts to myofibroblasts, thereby inhibiting excessive scar formation.
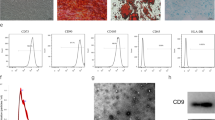
The WNT/β-catenin signaling pathway serves a vital role in proliferation, differentiation, movement, and morphology of cells, and it is also involved in mediating stem cell pluripotency [20]. β‐Catenin, a subunit of the cadherin protein complex, is an integral component of the classic WNT signaling pathway [42]. Accumulating evidence has confirmed that activation of the WNT/β‐catenin signaling pathway plays an essential role in the proliferative phase of wound healing [42, 43]. Our previous studies showed that WNT-responsive oral mucosal stem cells are activated in response to oral mucosal injury, thereby accelerating wound healing [21]. Thus, according to the conclusion that ADMSC-Exos activated WNT/β-catenin signaling pathway in this study, we have reason to deduce that whether ADMSC-Exos can also promote skin stem cell differentiation through the WNT signaling pathway during wound healing remains to be further studied. Moreover, our previous studies also showed that WNT-responsive stem cells can regulate periodontal membrane fibrosis and alveolar bone density to adapt to changes in mechanical stress [22]. Therefore, it remains to be further studied whether the effect of ADMSC-Exos on skin wound healing can also regulate the effect of mechanical tension by regulating WNT-responsive stem cells, which would be significant. At present, exosomes are an emerging platform and a critical factor in facilitating WNT secretion and transport. Zhang et al. found that exosomes from human umbilical cord MSCs can deliver WNT4 to target cells and activate β-catenin nuclear translocation, which has a positive effect on wound healing [44]. Ma et al. found that ADMSC-Exos could increase the protein expression of WNT and β-catenin in HaCaT cells treated with hydrogen peroxide to simulate skin damage[35]. However, their study did not research the effect of exosomes on fibroblasts during wound healing, and did not investigate the expression of ECM. Our study just complemented this and further refined the mechanism of exosomes through WNT signaling pathway. In this study, the elevated expression of β‐catenin in vitro and in vivo under treatment with ADMSC-Exos suggested that WNT/β‐catenin signaling may be related to the underlying mechanism of ADMSC‐Exos in wound healing. However, because of the complexity of wound tissue healing, more studies should be carried out on the association between the WNT/β-catenin signaling pathway and cutaneous wound healing.
Conclusion
In summary, ADMSC-Exos implies a pivotally stimulative role for wound healing process, in which this study revealed that WNT/β-catenin signaling pathway was involved. Furthermore, ADMSC-Exos had a preventive effect on cicatrization by boosting the proliferation and migration of fibroblasts, downregulating the expression of fibroblast α-SMA, and promoting the rearrangement of collagen fibres concurrently, which showed great possibilities for wound applications, providing novel treatment strategies for wound healing.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Code Availability
Not applicable.
References
Blakytny, R., & Jude, E. (2006). The molecular biology of chronic wounds and delayed healing in diabetes. Diabetic Medicine, 23(6), 594–608.
Da, L. C., Huang, Y. Z., & **e, H. Q. (2017). Progress in development of bioderived materials for dermal wound healing. Regen Biomater, 4(5), 325–334.
Darby, I. A., Laverdet, B., Bonté, F., & Desmoulière, A. (2014). Fibroblasts and myofibroblasts in wound healing. Clinical, Cosmetic and Investigational Dermatology, 7, 301–311.
Jiang, D., & Scharffetter-Kochanek, K. (2020). Mesenchymal Stem Cells Adaptively Respond to Environmental Cues Thereby Improving Granulation Tissue Formation and Wound Healing. Front Cell Dev Biol, 8, 697.
Vedrenne, N., Coulomb, B., Danigo, A., Bonté, F., & Desmoulière, A. (2012). The complex dialogue between (myo)fibroblasts and the extracellular matrix during skin repair processes and ageing. Pathologie Biologie, 60(1), 20–27.
Castilla, D. M., Liu, Z. J., Tian, R., Li, Y., Livingstone, A. S., & Velazquez, O. C. (2012). A novel autologous cell-based therapy to promote diabetic wound healing. Annals of Surgery, 256(4), 560–572.
Si, Z., Wang, X., Sun, C., Kang, Y., Xu, J., Wang, X., et al. (2019). Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomedicine and Pharmacotherapy, 114, 108765.
Shukla, L., Yuan, Y., Shayan, R., Greening, D. W., & Karnezis, T. (2020). Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Frontiers in Pharmacology, 11, 158.
Tsiapalis, D., & O’Driscoll, L. (2020). Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells, 9(4), 991.
Isakson, M., de Blacam, C., Whelan, D., McArdle, A., & Clover, A. J. (2015). Mesenchymal Stem Cells and Cutaneous Wound Healing: Current Evidence and Future Potential. Stem Cells International, 2015, 831095.
Saheli, M., Bayat, M., Ganji, R., Hendudari, F., Kheirjou, R., Pakzad, M., et al. (2020). Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Archives for Dermatological Research. Archiv für Dermatologische Forschung, 312(5), 325–336.
Wu, Y., Peng, Y., Gao, D., Feng, C., Yuan, X., Li, H., et al. (2015). Mesenchymal stem cells suppress fibroblast proliferation and reduce skin fibrosis through a TGF-β3-dependent activation. International Journal of Lower Extremity Wounds, 14(1), 50–62.
Ebrahimi, M., & Botelho, M. (2017). Adult Stem Cells of Orofacial Origin: Current Knowledge and Limitation and Future Trend in Regenerative Medicine. Tissue Eng Regen Med, 14(6), 719–733.
Costa, M. H. G., McDevitt, T. C., Cabral, J. M. S., da Silva, C. L., & Ferreira, F. C. (2017). Tridimensional configurations of human mesenchymal stem/stromal cells to enhance cell paracrine potential towards wound healing processes. Journal of Biotechnology, 262, 28–39.
Kuo, Y. R., Wang, C. T., Cheng, J. T., Kao, G. S., Chiang, Y. C., & Wang, C. J. (2016). Adipose-Derived Stem Cells Accelerate Diabetic Wound Healing Through the Induction of Autocrine and Paracrine Effects. Cell Transplantation, 25(1), 71–81.
**g, W., Zhiguo, W., **a, C., Kun, L., Rongan, H., & Yu, A. (2019). Research Progress on Exosomes Derived from Human Adipose Mesenchymal Stem Cells. International Journal of Sciences, 8(03), 114–117.
Gruenberg, J., & van der Goot, F. G. (2006). Mechanisms of pathogen entry through the endosomal compartments. Nature Reviews: Molecular Cell Biology, 7(7), 495–504.
Fang, Y., Zhang, Y., Zhou, J., & Cao, K. (2019). Adipose-derived mesenchymal stem cell exosomes: A novel pathway for tissues repair. Cell and Tissue Banking, 20(2), 153–161.
Aryani, A., & Denecke, B. (2016). Exosomes as a Nanodelivery System: A Key to the Future of Neuromedicine? Molecular Neurobiology, 53(2), 818–834.
Igota, S., Tosa, M., Murakami, M., Egawa, S., Shimizu, H., Hyakusoku, H., et al. (2013). Identification and characterization of Wnt signaling pathway in keloid pathogenesis. International Journal of Medical Sciences, 10(4), 344–354.
Yuan, X., Xu, Q., Zhang, X., Van Brunt, L. A., Ticha, P., & Helms, J. A. (2019). Wnt-Responsive Stem Cell Fates in the Oral Mucosa. iScience, 21, 84–94.
Xu, Q., Yuan, X., Zhang, X., Chen, J., Shi, Y., Brunski, J. B., et al. (2019). Mechanoadaptive Responses in the Periodontium Are Coordinated by Wnt. Journal of Dental Research, 98(6), 689–697.
Cui, X., He, Z., Liang, Z., Chen, Z., Wang, H., & Zhang, J. (2017). Exosomes From Adipose-derived Mesenchymal Stem Cells Protect the Myocardium Against Ischemia/Reperfusion Injury Through Wnt/β-Catenin Signaling Pathway. Journal of Cardiovascular Pharmacology, 70(4), 225–231.
Wang, J., Cai, X., Wang, Z. G., Xu, Q. C., Li, K., & Hua, C. (2019). Isolation and identification of exosomes from human adipose-derived mesenchymal. Chinese Journal of Tissue Engineering Research, 23, 2651–2658.
van der Pol, E., Böing, A. N., Harrison, P., Sturk, A., & Nieuwland, R. (2012). Classification, functions, and clinical relevance of extracellular vesicles. Pharmacological Reviews, 64(3), 676–705.
Gurtner, G. C., Werner, S., Barrandon, Y., & Longaker, M. T. (2008). Wound repair and regeneration. Nature, 453(7193), 314–321.
van Niel, G., D’Angelo, G., & Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews: Molecular Cell Biology, 19(4), 213–228.
Tian, M., Ticer, T., Wang, Q., Walker, S., Pham, A., Suh, A., et al. (2020). Adipose-Derived Biogenic Nanoparticles for Suppression of Inflammation. Small, 16(10), e1904064.
Andaloussi, S. E. L., Mäger, I., Breakefield, X. O., & Wood, M. J. (2013). Extracellular vesicles: biology and emerging therapeutic opportunities. Nature Reviews Drug Discovery, 12(5), 347–357.
Wu, P., Zhang, B., Shi, H., Qian, H., & Xu, W. (2018). MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy, 20(3), 291–301.
Shabbir, A., Cox, A., Rodriguez-Menocal, L., Salgado, M., & Van Badiavas, E. (2015). Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells and Development, 24(14), 1635–1647.
Geiger, A., Walker, A., & Nissen, E. (2015). Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochemical and Biophysical Research Communications, 467(2), 303–309.
Tang, Y. T., Huang, Y. Y., Zheng, L., Qin, S. H., Xu, X. P., An, T. X., et al. (2017). Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. International Journal of Molecular Medicine, 40(3), 834–844.
Hu, L., Wang, J., Zhou, X., **ong, Z., Zhao, J., Yu, R., et al. (2016). Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Scientific Reports, 6, 32993.
Ma, T., Fu, B., Yang, X., **ao, Y., & Pan, M. (2019). Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. Journal of Cellular Biochemistry, 120(6), 10847–10854.
Kallmeyer, K., André-Lévigne, D., Baquié, M., Krause, K. H., Pepper, M. S., Pittet-Cuénod, B., et al. (2020). Fate of systemically and locally administered adipose-derived mesenchymal stromal cells and their effect on wound healing. Stem Cells Translational Medicine, 9(1), 131–144.
Driskell, R. R., Lichtenberger, B. M., Hoste, E., Kretzschmar, K., Simons, B. D., Charalambous, M., et al. (2013). Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature, 504(7479), 277–281.
Thulabandu, V., Chen, D., Atit, R. P. (2018). Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev Dev Biol 7(2), https://doi.org/10.1002/wdev.307
Wang, L., Hu, L., Zhou, X., **ong, Z., Zhang, C., Shehada, H. M. A., et al. (2017). Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Scientific Reports, 7(1), 13321.
Karppinen, S. M., Heljasvaara, R., Gullberg, D., Tasanen, K., Pihlajaniemi, T. (2019). Toward understanding scarless skin wound healing and pathological scarring. F1000Res, 8. F1000 Faculty Rev-787.
Hinz, B., Phan, S. H., Thannickal, V. J., Prunotto, M., Desmoulière, A., Varga, J., et al. (2012). Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. American Journal of Pathology, 180(4), 1340–1355.
Leavitt, T., Hu, M. S., Marshall, C. D., Barnes, L. A., Lorenz, H. P., & Longaker, M. T. (2016). Scarless wound healing: Finding the right cells and signals. Cell and Tissue Research, 365(3), 483–493.
Wang, X., Zhu, Y., Sun, C., Wang, T., Shen, Y., Cai, W., et al. (2017). Feedback Activation of Basic Fibroblast Growth Factor Signaling via the Wnt/β-Catenin Pathway in Skin Fibroblasts. Frontiers in Pharmacology, 8, 32.
Zhang, B., Wu, X., Zhang, X., Sun, Y., Yan, Y., Shi, H., et al. (2015). Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Translational Medicine, 4(5), 513–522.
Acknowledgements
The authors wish to thank all the staff of the Burn and Plastic Surgery Department of the Affiliated Hospital of Qingdao University for their support of this study.
Funding
This paper was supported by the Shandong Provincial Natural Science Foundation (Grant/Award Number: ZR2017MH083, ZR2020MH183).
Author information
Authors and Affiliations
Contributions
Cong Li, Yu An: Conception and design, experiments, manuscript writing, data analysis and interpretation; Yu Sun, Fan Yang: Experiments, Collection of data; Zhiguo Wang, Quanchen Xu: Conception and design, manuscript writing, final approval of manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The Ethics Committee of the Affiliated Hospital of Qingdao University approved the study (QYFY WZLL 26708), and it was conducted in accordance with the Declaration of Helsinki.
Consent to Participate
The fat tissues and foreskin tissue were collected after obtaining informed consent from the donors.
Consent for Publication
All authors consented to publish this paper.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cong Li and Yu An contributed equally to this work.
Cong Li and Yu An are co-first authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, C., An, Y., Sun, Y. et al. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote Wound Healing Through the WNT/β-catenin Signaling Pathway in Dermal Fibroblasts. Stem Cell Rev and Rep 18, 2059–2073 (2022). https://doi.org/10.1007/s12015-022-10378-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10378-0