Abstract
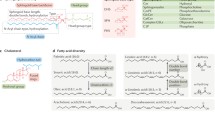
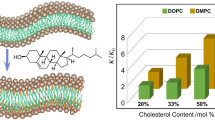
Lipid composition determines membrane properties, and cholesterol plays a major role in this determination as it regulates membrane fluidity and permeability, as well as induces the formation of coexisting phases and domains in the membrane. Biological membranes display a very diverse lipid composition, the lateral organization of which plays a crucial role in regulating a variety of membrane functions. We hypothesize that, during biological evolution, membranes with a particular cholesterol content were selected to perform certain functions in the cells of eukaryotic organisms. In this review, we discuss the major membrane properties induced by cholesterol, and their relationship to certain membrane functions.







Similar content being viewed by others
References
Roux, B., Berneche, S., Egwolf, B., Lev, B., Noskov, S. Y., Rowley, C. N., et al. (2011). Ion selectivity in channels and transporters. The Journal of General Physiology, 137, 415–426.
Hille, B. (2001). Ion Channels of Excitable Membranes. (3rd Edition). Sinauer Associates Inc, Sunderland, MA.
Luckey, M. (2008). Membrane Structural Biology: with Biochemical and Biophysical Foundations. Cambridge University Press, New York, NY.
Diamond, J. M., & Katz, Y. (1974). Interpretation of nonelectrolyte partition coefficients between dimyristoyl lecithin and water. Journal of Membrane Biology, 17, 121–154.
Viola, A. (2001). The amplification of TCR signaling by dynamic membrane microdomains. Trends in Immunology, 22, 322–327.
Simons, K., & Toomre, D. (2000). Lipid rafts and signal transduction. Nature Reviews. Molecular Cell Biology, 1, 31–39.
Kusumi, A., Fujiwara, T. K., Morone, N., Yoshida, K. J., Chadda, R., **e, M., et al. (2012). Membrane mechanisms for signal transduction: the coupling of the meso-scale raft domains to membrane-skeleton-induced compartments and dynamic protein complexes. Seminars in Cell and Developmental Biology, 23, 126–144.
Nelson, D. L., & Cox, M. M. (2008). Lehninger Principles of Biochemistry. 5th edition. W. H. Freeman and Company, New York, NY.
Borchman, D., & Yappert, M. C. (2010). Lipids and the ocular lens. Journal of Lipid Research, 51, 2473–2488.
Subczynski, W. K., Raguz, M., Widomska, J., Mainali, L., & Konovalov, A. (2012). Functions of cholesterol and the cholesterol bilayer domain specific to the fiber-cell plasma membrane of the eye lens. Journal of Membrane Biology, 245, 51–68.
Epand, R. M. (2005). Role of membrane lipids in modulating the activity of membrane-bound enzymes. In P. L. Yeagle (Ed.), The Structure of Biological Membrane (pp. 499–509). Boca Raton: CRC Press.
Reichow, S. L., & Gonen, T. (2009). Lipid-protein interactions probed by electron crystallography. Current Opinion in Structural Biology, 19, 560–565.
Tong, J., Briggs, M. M., & McIntosh, T. J. (2012). Water permeability of aquaporin-4 channel depends on bilayer composition, thickness, and elasticity. Biophysical Journal, 103, 1899–1908.
Tong, J., Canty, J. T., Briggs, M. M., & McIntosh, T. J. (2013). The water permeability of lens aquaporin-0 depends on its lipid bilayer environment. Experimental Eye Research, 113, 32–40.
Bloch, K. E. (1983). Sterol structure and membrane function. CRC Critical Reviews in Biochemistry, 14, 47–92.
Bloom, M., & Mouritsen, O. G. (1995). The evolution of membrane. In R. Lipowsky, E. Sackmann (eds.). Structure and Dynamics of Membrane (pp. 65–95). Amsterdam: Elsevier.
Miao, L., Nielsen, M., Thewalt, J., Ipsen, J. H., Bloom, M., Zuckermann, M. J., et al. (2002). From lanosterol to cholesterol: structural evolution and differential effects on lipid bilayers. Biophysical Journal, 82, 1429–1444.
Rog, T., Pasenkiewicz-Gierula, M., Vattulainen, I., & Karttunen, M. (2007). What happens if cholesterol is made smoother: importance of methyl substituents in cholesterol ring structure on phosphatidylcholine-sterol interaction. Biophysical Journal, 92, 3346–3357.
Hannich, J. T., Umebayashi, K., & Riezman, H. (2011). Distribution and functions of sterols and sphingolipids. Cold Spring Harbor Perspectives in Biology, 3, 1–14.
Bretscher, M. S., & Munro, S. (1993). Cholesterol and the Golgi apparatus. Science (New York, N.Y.), 261, 1280–1281.
Schroeder, F., Frolov, A. A., Murphy, E. J., Atshaves, B. P., Jefferson, J. R., Pu, L., et al. (1996). Recent advances in membrane cholesterol domain dynamics and intracellular cholesterol trafficking. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine, 213, 150–177.
van Meer, G., Voelker, D. R., & Feigenson, G. W. (2008). Membrane lipids: where they are and how they behave. Nature reviews. Molecular Cell Biology, 9, 112–124.
Spector, A. A., & Yorek, M. A. (1985). Membrane lipid composition and cellular function. Journal of Lipid Research, 26, 1015–1035.
Shinitzky, M., & Barenholz, Y. (1978). Fluidity parameters of lipid regions determined by fluorescence polarization. Biochimica et Biophysica Acta, 515, 367–394.
Mainali, L., Raguz, M., & Subczynski, W. K. (2013). Formation of cholesterol bilayer domains precedes formation of cholesterol crystals in cholesterol/dimyristoylphosphatidylcholine membranes: EPR and DSC studies. Journal of Physical Chemistry B, 117, 8994–9003.
Raguz, M., Mainali, L., Widomska, J., & Subczynski, W. K. (2011). Using spin-label electron paramagnetic resonance (EPR) to discriminate and characterize the cholesterol bilayer domain. Chemistry and Physics of Lipids, 164, 819–829.
Li, L. K., So, L., & Spector, A. (1987). Age-dependent changes in the distribution and concentration of human lens cholesterol and phospholipids. Biochimica et Biophysica Acta, 917, 112–120.
Truscott, R. J. (2000). Age-related nuclear cataract: a lens transport problem. Ophthalmic Research, 32, 185–194.
Li, L. K., So, L., & Spector, A. (1985). Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. Journal of Lipid Research, 26, 600–609.
Mason, R. P., & Jacob, R. F. (2003). Membrane microdomains and vascular biology. Emerging role in atherogenesis. Circulation, 107, 2270–2273.
Mason, R., Tulenko, T. N., & Jacob, R. F. (2003). Direct evidence for cholesterol crystalline domains in biological membranes: role in human pathobiology. Biochimica et Biophysica Acta, 1610, 198–207.
Jacob, R. F., Cenedella, R. J., & Mason, R. P. (1999). Direct evidence for immiscible cholesterol domains in human ocular lens fiber cell plasma membranes. Journal of Biological Chemistry, 274, 31613–31618.
Singer, S. J., & Nicolson, G. L. (1972). The fluid mosaic model of the structure of cell membranes. Science (New York, N.Y.), 175, 720–731.
Kusumi, A., Suzuki, K. G., Kasai, R. S., Ritchie, K., & Fujiwara, T. K. (2011). Hierarchical mesoscale domain organization of the plasma membrane. Trends in Biochemical Sciences, 36, 604–615.
Kusumi, A., Fujiwara, T. K., Chadda, R., **e, M., Tsunoyama, T. A., Kalay, Z., et al. (2012). Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of Singer and Nicolson’s fluid-mosaic model. Annual Review of Cell and Developmental Biology, 28, 215–250.
Wisniewska, A., Draus, J., & Subczynski, W. K. (2003). Is a fluid-mosaic model of biological membranes fully relevant? Studies on lipid organization in model and biological membranes. Cellular and Molecular Biology Letters, 8, 147–159.
Nicolson, G. L. (2014). The fluid-mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochimica et Biophysica Acta, 1838, 1451–1466.
Subczynski, W. K., Antholine, W. E., Hyde, J. S., & Kusumi, A. (1990). Microimmiscibility and three-dimensional dynamic structures of phosphatidylcholine-cholesterol membranes: translational diffusion of a copper complex in the membrane. Biochemistry, 29, 7936–7945.
Subczynski, W. K., Wisniewska, A., Hyde, J. S., & Kusumi, A. (2007). Three-dimensional dynamic structure of the liquid-ordered domain in lipid membranes as examined by pulse-EPR oxygen probing. Biophysical Journal, 92, 1573–1584.
Subczynski, W. K., Hyde, J. S., & Kusumi, A. (1991). Effect of alkyl chain unsaturation and cholesterol intercalation on oxygen transport in membranes: a pulse ESR spin labeling study. Biochemistry, 30, 8578–8590.
Almeida, P. F., Pokorny, A., & Hinderliter, A. (2005). Thermodynamics of membrane domains. Biochimica et Biophysica Acta, 1720, 1–13.
Heberle, F. A., & Feigenson, G. W. (2011). Phase separation in lipid membranes. Cold Spring Harbor. Perspectives in Biology, 3, 1–13.
Simons, K., & Vaz, W. L. (2004). Model systems, lipid rafts, and cell membranes. Annual Review of Biophysics and Biomolecular Structure, 33, 269–295.
Huang, J., Buboltz, J. T., & Feigenson, G. W. (1999). Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochimica et Biophysica Acta, 1417, 89–100.
Marsh, D. (1981). Electron spin resonance: spin labels. In E. Grell (ed.), Membrane Spectroscopy (pp. 51–142). Berlin: Springer.
Devaux, P. F. (1983). ESR and NMR studies of lipid-protein interactions in membranes. In L. J. Berliner, J. Reuben (eds.), Biological Magnetic Resonance (pp. 183–299). New York: Plenum Press.
Schreier, S., Polnaszek, C. F., & Smith, I. C. (1978). Spin labels in membranes. Problems in practice. Biochimica et Biophysica Acta, 515, 395–436.
Rog, T., & Pasenkiewicz-Gierula, M. (2001). Cholesterol effects on the phosphatidylcholine bilayer nonpolar region: a molecular simulation study. Biophysical Journal, 81, 2190–2202.
Mainali, L., Feix, J. B., Hyde, J. S., & Subczynski, W. K. (2011). Membrane fluidity profiles as deduced by saturation-recovery EPR measurements of spin-lattice relaxation times of spin labels. Journal of Magnetic Resonance, 212, 418–425.
Mainali, L., Hyde, J. S., & Subczynski, W. K. (2013). Using spin-label W-band EPR to study membrane fluidity profiles in samples of small volume. Journal of Magnetic Resonance, 226, 35–44.
Robinson, B. H., Haas, D. A., & Mailer, C. (1994). Molecular dynamics in liquids: spin-lattice relaxation of nitroxide spin labels. Science (New York, N.Y.), 263, 490–493.
Mailer, C., Nielsen, R. D., & Robinson, B. H. (2005). Explanation of spin-lattice relaxation rates of spin labels obtained with multifrequency saturation recovery EPR. The Journal of Physical Chemistry. A, 109, 4049–4061.
Rog, T., & Pasenkiewicz-Gierula, M. (2004). Non-polar interactions between cholesterol and phospholipids: a molecular dynamics simulation study. Biophysical Chemistry, 107, 151–164.
Rog, T., & Pasenkiewicz-Gierula, M. (2001). Cholesterol effects on the phospholipid condensation and packing in the bilayer: a molecular simulation study. FEBS Letters, 502, 68–71.
Murzyn, K., Rog, T., Jezierski, G., Takaoka, Y., & Pasenkiewicz-Gierula, M. (2001). Effects of phospholipid unsaturation on the membrane/water interface: a molecular simulation study. Biophysical Journal, 81, 170–183.
Plesnar, E., Subczynski, W. K., & Pasenkiewicz-Gierula, M. (2013). Comparative computer simulation study of cholesterol in hydrated unary and binary lipid bilayers and in an anhydrous crystal. The Journal of Physical Chemistry. B, 117, 8758–8769.
Griffith, O. H., Dehlinger, P. J., & Van, S. P. (1974). Shape of the hydrophobic barrier of phospholipid bilayers (evidence for water penetration in biological membranes). Journal of Membrane Biology, 15, 159–192.
Griffith, O. H., & Jost, P. C. (1976). Lipid spin labels in biological membranes. In L. J. Berliner (ed.), Spin Labeling Theory and Applications (pp. 453–523). New York, San Francisco, London: Academic Press.
Subczynski, W. K., Wisniewska, A., Yin, J.-J., Hyde, J. S., & Kusumi, A. (1994). Hydrophobic barriers of lipid bilayer membranes formed by reduction of water penetration by alkyl chain unsaturation and cholesterol. Biochemistry, 33, 7670–7681.
Wisniewska, A., & Subczynski, W. K. (1998). Effects of polar carotenoids on the shape of the hydrophobic barrier of phospholipid bilayers. Biochimica et Biophysica Acta, 1368, 235–246.
Kusumi, A., Subczynski, W. K., & Hyde, J. S. (1982). Oxygen transport parameter in membranes as deduced by saturation recovery measurements of spin-lattice relaxation times of spin labels. Proceedings of National Academic Sciences USA, 79, 1854–1858.
Subczynski, W. K., Hyde, J. S., & Kusumi, A. (1989). Oxygen permeability of phosphatidylcholine-cholesterol membranes. Proceedings National Academic Sciences USA, 86, 4474–4478.
Ashikawa, I., Yin, J.-J., Subczynski, W. K., Kouyama, T., Hyde, J. S., & Kusumi, A. (1994). Molecular organization and dynamics in bacteriorhodopsin-rich reconstituted membranes: discrimination of lipid environments by the oxygen transport parameter using a pulse ESR spin-labeling technique. Biochemistry, 33, 4947–4952.
Subczynski, W. K., Widomska, J., Wisniewska, A., & Kusumi, A. (2007). Saturation-recovery electron paramagnetic resonance discrimination by oxygen transport (DOT) method for characterizing membrane domains. In T. J. McIntosh (Ed.) Methods in Molecular Biology, Lipid Rafts (pp. 143–157). Humana Press: Totowa.
Mainali, L., Raguz, M., & Subczynski, W. K. (2011). Phase-separation and domain-formation in cholesterol-sphingomyelin mixture: pulse-EPR oxygen probing. Biophysical Journal, 101, 837–846.
Subczynski, W. K., Raguz, M., & Widomska, J. (2010). Studying lipid organization in biological membranes using liposomes and EPR spin labeling. Methods in Molecular Biology, 606, 247–269.
Kawasaki, K., Yin, J.-J., Subczynski, W. K., Hyde, J. S., & Kusumi, A. (2001). Pulse EPR detection of lipid exchange between protein rich raft and bulk domains in the membrane: methodology development and its application to studies of influenza viral membrane. Biophysical Journal, 80, 738–748.
Raguz, M., Mainali, L., Widomska, J., & Subczynski, W. K. (2011). The immiscible cholesterol bilayer domain exists as an integral part of phospholipid bilayer membranes. Biochimica et Biophysica Acta, 1808, 1072–1080.
Mitchell, P. (1961). Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature, 191, 144–148.
Mannella, C. A. (2006). Structure and dynamics of the mitochondrial inner membrane cristae. Biochimica et Biophysica Acta, 1763, 542–548.
Schlame, M., Brody, S., & Hostetler, K. Y. (1993). Mitochondrial cardiolipin in diverse eukaryotes. Comparison of biosynthetic reactions and molecular acyl species. European Journal of Biochemistry, 212, 727–735.
Schlame, M., Horvath, L., & Vigh, L. (1990). Relationship between lipid saturation and lipid-protein interaction in liver mitochondria modified by catalytic hydrogenation with reference to cardiolipin molecular species. The Biochemical Journal, 265, 79–85.
Simons, K., & Ikonen, E. (1997). Functional rafts in cell membranes. Nature, 387, 569–572.
Zurzolo, C., van Meer, G., & Mayor, S. (2003). The order of rafts. Conference on microdomains, lipid rafts and caveolae. EMBO Reports, 4, 1117–1121.
Subczynski, W. K., & Kusumi, A. (2003). Dynamics of raft molecules in the cell and artificial membranes: approaches by pulse EPR spin labeling and single molecule optical microscopy. Biochimica et Biophysica Acta, 1610, 231–243.
Mayor, S., & Rao, M. (2004). Rafts: scale-dependent, active lipid organization at the cell surface. Traffic (Copenhagen, Denmark), 5, 231–240.
Mukherjee, S., & Maxfield, F. R. (2004). Membrane domains. Annual Review of Cell and Developmental Biology, 20, 839–866.
Kusumi, A., Koyama-Honda, I., & Suzuki, K. (2004). Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic (Copenhagen, Denmark), 5, 213–230.
Kusumi, A., Nakada, C., Ritchie, K., Murase, K., Suzuki, K., Murakoshi, H., et al. (2005). Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annual Review of Biophysics and Biomolecular Structure, 34, 351–378.
Kusumi, A., Ike, H., Nakada, C., Murase, K., & Fujiwara, T. (2005). Single-molecule tracking of membrane molecules: plasma membrane compartmentalization and dynamic assembly of raft-philic signaling molecules. Seminars in Immunology, 17, 3–21.
Munro, S. (2003). Lipid rafts: elusive or illusive? Cell, 115, 377–388.
Taner, S. B., Onfelt, B., Pirinen, N. J., McCann, F. E., Magee, A. I., & Davis, D. M. (2004). Control of immune responses by trafficking cell surface proteins, vesicles and lipid rafts to and from the immunological synapse. Traffic (Copenhagen, Denmark), 5, 651–661.
van Meer, G., & Sprong, H. (2004). Membrane lipids and vesicular traffic. Current Opinion in Cell Biology, 16, 373–378.
Plesnar, E., Subczynski, W. K., & Pasenkiewicz-Gierula, M. (2012). Saturation with cholesterol increases vertical order and smoothes the surface of the phosphatidylcholine bilayer: a molecular simulation study. Biochimica et Biophysica Acta, 1818, 520–529.
Kusumi, A., & Pasenkiewicz-Gierula, M. (1988). Rotational diffusion of a steroid molecule in phosphatidylcholine membranes: effects of alkyl chain length, unsaturation, and cholesterol as studied by a spin-label method. Biochemistry, 27, 4407–4415.
Subczynski, W. K., Hopwood, L. E., & Hyde, J. S. (1992). Is the mammalian cell plasma membrane a barrier to oxygen transport? Journal of General Physiology, 100, 69–87.
Rog, T., & Pasenkiewicz-Gierula, M. (2006). Cholesterol-sphingomyelin interactions: a molecular dynamics simulation study. Biophysical Journal, 91, 3756–3767.
Harding, J. J. (1997). Biochemistry of the eye. In J. J. Harding (Ed.), Lens (pp. 94–135). London: Chapman and Hall.
Lynnerup, N., Kjeldsen, H., Heegaard, S., Jacobsen, C., & Heinemeier, J. (2008). Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PloS One, 3, e1529.
Mainali, L., Raguz, M., O’Brien, W.J., & Subczynski, W.K. (2016). Changes in the properties and organization of human lens lipid membranes occurring with age. Current Eye Research, doi:10.1080/02713683.02712016.01231325.
Yappert, M. C., Rujoi, M., Borchman, D., Vorobyov, I., & Estrada, R. (2003). Glycero- versus sphingo-phospholipids: correlations with human and non-human mammalian lens growth. Experimental Eye Research, 76, 725–734.
Rujoi, M., **, J., Borchman, D., Tang, D., & Yappert, M. C. (2003). Isolation and lipid characterization of cholesterol-enriched fractions in cortical and nuclear human lens fibers. Investigative Ophthalmology and Visual Science, 44, 1634–1642.
Mainali, L., Raguz, M., O’Brien, W. J., & Subczynski, W. K. (2015). Properties of membranes derived from the total lipids extracted from clear and cataractous lenses of 61-70-year-old human donors. European Biophysics Journal, 44, 91–102.
Duewell, P., Kono, H., Rayner, K. J., Sirois, C. M., Vladimer, G., Bauernfeind, F. G., et al. (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature, 464, 1357–1361.
Jacob, R. F., Cenedella, R. J., & Mason, R. P. (2001). Evidence for distinct cholesterol domains in fiber cell membranes from cataractous human lenses. Journal of Biological Chemistry, 276, 13573–13578.
Kusumi, A., Tsuda, M., Akino, T., Ohnishi, S., & Terayama, Y. (1983). Protein-phospholipid-cholesterol interaction in the photolysis of invertebrate rhodopsin. Biochemistry, 22, 1165–1170.
Kusumi, A., Subczynski, W. K., Pasenkiewicz-Gierula, M., Hyde, J. S., & Merkle, H. (1986). Spin-label studies on phosphatidylcholine-cholesterol membranes: effects of alkyl chain length and unsaturation in the fluid phase. Biochimica et Biophysica Acta, 854, 307–317.
Subczynski, W. K., & Wisniewska, A. (2000). Physical properties of lipid bilayer membranes: relevance to membrane biological functions. Acta Biochimica Polonica, 47, 613–625.
Rog, T., & Pasenkiewicz-Gierula, M. (2006). Cholesterol effects on a mixed-chain phosphatidylcholine bilayer: a molecular dynamics simulation study. Biochimie, 88, 449–460.
Skulachev, V. P. (1990). Power transmission along biological membranes. Journal of Membrane Biology, 114, 97–112.
Raguz, M., Widomska, J., Dillon, J., Gaillard, E. R., & Subczynski, W. K. (2009). Physical properties of the lipid bilayer membrane made of cortical and nuclear bovine lens lipids: EPR spin-labeling studies. Biochimica et Biophysica Acta, 1788, 2380–2388.
Loura, L. M., Fedorov, A., & Prieto, M. (2001). Fluid-fluid membrane microheterogeneity: a fluorescence resonance energy transfer study. Biophysical Journal, 80, 776–788.
Acknowledgements
This work was supported by grants EY015526, EB001980, and EY001931 from the National Institutes of Health, USA. Faculty of Biochemistry, Biophysics, and Biotechnology of Jagiellonian University is a partner of the Leading National Research Center (KNOW) supported by the Ministry of Science and Higher Education, Poland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Subczynski, W.K., Pasenkiewicz-Gierula, M., Widomska, J. et al. High Cholesterol/Low Cholesterol: Effects in Biological Membranes: A Review. Cell Biochem Biophys 75, 369–385 (2017). https://doi.org/10.1007/s12013-017-0792-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-017-0792-7